Update in Thymoma for Surgical Pathologists
Ruchira Ruangchira-urai, MD1, Jitsupa Treetipsatit, MD1
Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University,
Bangkok 10700 Thailand
Corresponding author: Jitsupa Treetipsatit, MD
Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University
2 Prannok Road, Bangkok Noi,
Bangkok 10700 Thailand Email: jitsupa.tre@mahidol.ac.th Tel: 662-419-6520
Received 25th October 2015; Accepted 25th November 2015
ABSTRACT
Thymoma is the most common mediastinal tumor in adulthood. By definition, it is a thymic epithelial neoplasm that displays at least some characteristics of normal thymus. Microscopically it comprises an admixture of thymic epithelial cells and immature T lymphocytes in various proportions and demonstrates marked intratumoral heterogeneity, which results in several histological subtypes. Recent studies have shown that thymoma is a neoplasm of at least low malignant potential and its prognosis mainly depends on disease stage and resectability status. Despite a controversy regarding histological subtype and its clinical relevance, recent data have shown that certain thymoma histological classifications might have a role in predicting survival and prognosis. The aim of this review will be an overview concept of thymomas that is intended to be informative and practical for daily surgical pathology practice. Particular emphasis is paid to the latest developments in the disease staging systems, histological classifications and specimen handling and reporting guidelines.
Keywords: Thymoma, Thymic epithelial neoplasm, Mediastinum, Specimen handling, Reporting guideline, Staging, Histological classification
OVERVIEW CONCEPT OF THYMOMA
Definition
Thymoma is a thymic epithelial neoplasm that demonstrates some organotypic features of normal thymus and is accompanied by variable numbers of reactive lymphoid cells. Organotypic features of normal thymus include (1) lobular architecture, (2) areas of medullary differentiation, (3) perivascular spaces, and (4) dual (epithelial-lymphoid) cell population with variable numbers of immature T lympho-cytes.1,2
Characteristic gross and histological features
Grossly, thymomas are well-circumscribed, solid tumors with fibrous encapsulation in the majority of cases, though; tumors lacking fibrous capsules may be encountered. Cut surfaces show lobulation with homogeneous, gray-white to light brown, rubbery tissue (Figure 1A). Cystic change as well as intratumoral hemorrhage can also be encountered (Figure 1B and 1C).
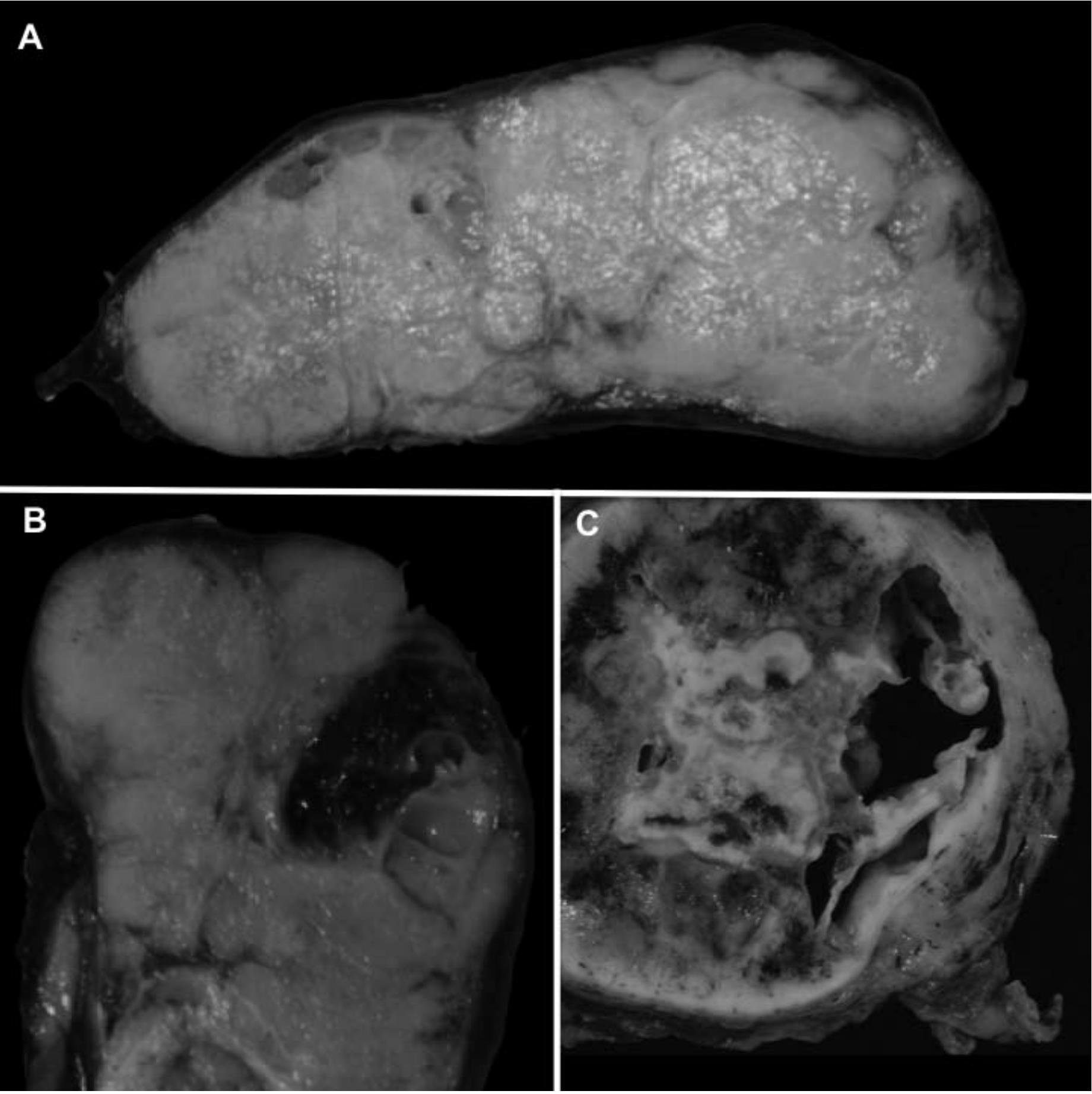
Figure 1 Gross features of thymomas. Typical gross appearance is well circumscribed with lobulation and homogeneous gray-white to light brown cut surface (A). Fibrous encapsulation of variable thickness is present. Focal intratumoral hemorrhage can also be noted (B). Occasionally, prominent cystic change is observed (C).
Microscopically, lobular architecture of thymomas is usually angulated in contour and is best appreciated on low magnification. The lobules are intervened by fibrous septa of varying thickness. The presence of an admixture of thymic epithelial cells and immature T lymphocytes in various proportions is a hallmark of thymomas. The thymic epithelial cells can be noted in 2 forms: (1) spindle to oval, and (2) round or polygonal. The spindle cells have small, elongated, or oval nuclei with dispersed chromatin. Nucleoli might be small and inconspicuous or absent. Cytoplasm is moderate in amount and lightly eosinophilic in appearance. The round or polygonal cells have vesicular nuclei containing conspicuous eosinophilic nucleoli and lightly eosinophilic or amphophilic cytoplasm usually with indistinct cell borders. Other organotypic features that can be encountered include areas of medullary differentiation which are characterized by well-delineated, rounded, pale foci within the tumor lobules that contain a decreased number of immature T lymphocytes and a greater proportion of epithelial cells and presence of perivascular spaces which are dilated empty spaces surrounding small vessels within the tumor in which reside variable numbers of lymphocytes. WHO Classification, the most widely used histological classification system, categorizes thymomas into several subtypes on a basis of the proportion of thymic epithelial cells to lymphocytes and features of the thymic epithelial cells. More details on the histological classification are provided in the section “Histological Classification” below.
In rare occasions, an unusual “sclerosing/ ancient thymoma” variant can be encountered. This variant of thymomas is characterized by extensive stromal hyalinization or sclerosis with only microscopic residual islands or streaks of thymic epithelial cells, which sometimes need to be highlighted by immunohistochemical markers for epithelial cells such as cytokeratins.3 It is important for surgical pathologists to be familiar with this entity in order to avoid misdiagnosing it as other diseases.
Immunohistochemistry
In general, the neoplastic epithelial cells in thymomas (both spindle and round/polygonal cells) usually show reactivity for keratins such as AE1/ AE3, low-molecular-weight cytokeratin (CAM5.2 and CK8/18), high-molecular-weight cytokeratin (34betaE12), CK19 and CK5/6.3,4 However, loss of keratin expression has been reported in some cases.5 A typical pattern of cytokeratin stain in thymomas is a lace-like pattern. Other epithelial markers such as BerEP4 and MOC31 can be positive in up to a third of thymomas.4 PAX8 (a transcription factor associated with the embryonic development of the thyroid, kidney, and the Mullerian system) expression has been reported in more than 50% of thymomas.6,7 p63 is positive in virtually all thymoma cases including the reported cases with loss of keratin expression.5,8,9 In contrast to thymic carcinoma, CD5 and CD117 expression is seen in a small subset of thymoma cases.4
Despite showing a similar staining pattern as described above, the spindle neoplastic epithelial cells can show positivity for markers not usually express in thymomas. For instance, CD20 and CK7 reactivity has been noted in 50 to 90% and 83% of thymomas with spindle neoplastic epithelial cells, respectively.10-12 In addition, focal staining of calretinin, synaptophysin, smooth muscle actin (SMA), and BCL2 has been reported in a significant subset of thymomas with spindle neoplastic epithelial cells as well as TTF-1 positivity in rare cases.12
The lymphocytic component of thymomas is mainly composed of immature T cells that usually express CD3, terminal deoxynucleotidyl transferase (TdT), CD1a, and CD99 (MIC2). Also there is a small subset of lymphocytes in thymomas that are CD20+ lymphocytes. These B cells can present either as scattered single cells or organized in lymphoid follicles with germinal centers.4
Clinical behavior
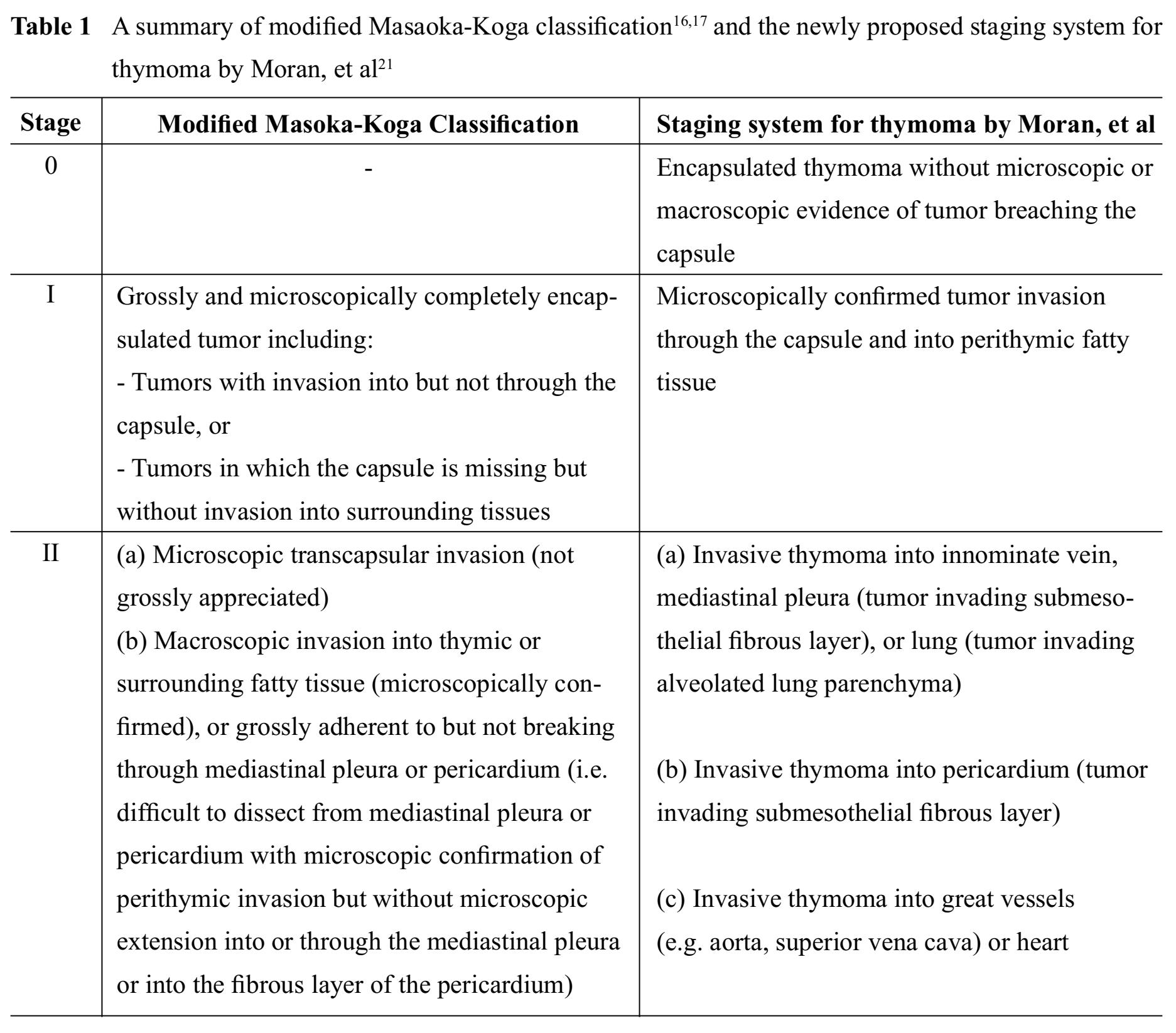
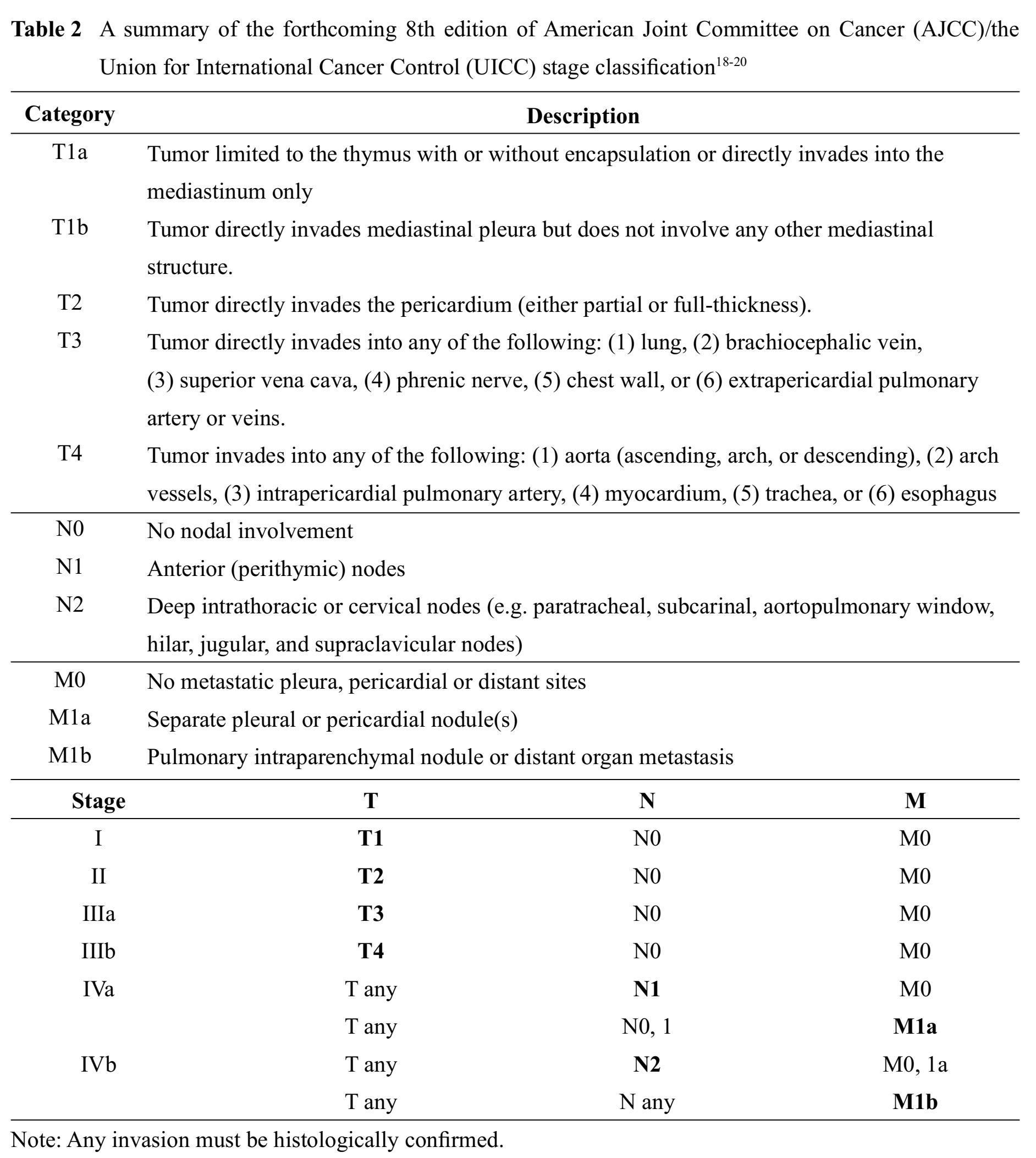
All thymomas, regardless of histological types, are now considered epithelial neoplasms of at least low malignant potential.1013 Clinical outcomes mainly depend on tumor invasiveness, which is represented by clinical staging, and status of resectability.1415 To date, there are several proposed staging systems for thymomas, most of which are based on a status of tumor capsule and tumor invasiveness. Staging systems that have been widely accepted in current practice include modified Masaoka-Koga classification1617 and the American Joint Committee on Cancer (AJCC)/the Union for International Cancer Control (UICC) stage classi-fication.18-20 Also there is a recently proposed staging system for thymoma by Moran, et al21, which is based on the largest and most representative cases and provide a clear definition of encapsulated versus invasive thymomas and extra-thymic tumor involvement.22 A recent study suggests that the newly proposed Moran staging system has prognostic strength comparable to the widely accepted modified Masao-ka-Koga classification.23
A summary of the aforementioned staging systems for thymoma is shown in Table 1 and 2.

RESECTION SPECIMEN HANDLING GUIDELINE
Grossing
If designated by a surgeon, orientation of anterior, posterior, left and right aspects of the specimen as well as identification of any important structures (such as pericardium, innominate vein, mediastinal pleural surface(s), or superior vena cava) that are attached to the resected tumor should be done before grossing. Once the different anatomical aspects and the attached important structures are identified and all of the key areas for assessment of resection margin status are inked, it is recommended that the tumor be serially sectioned from superior to inferior in a bread-loafing manner in order to expose the entire circumference of the tumor.24 Figure 2 illustrates a grossing process of thymoma resection specimens.
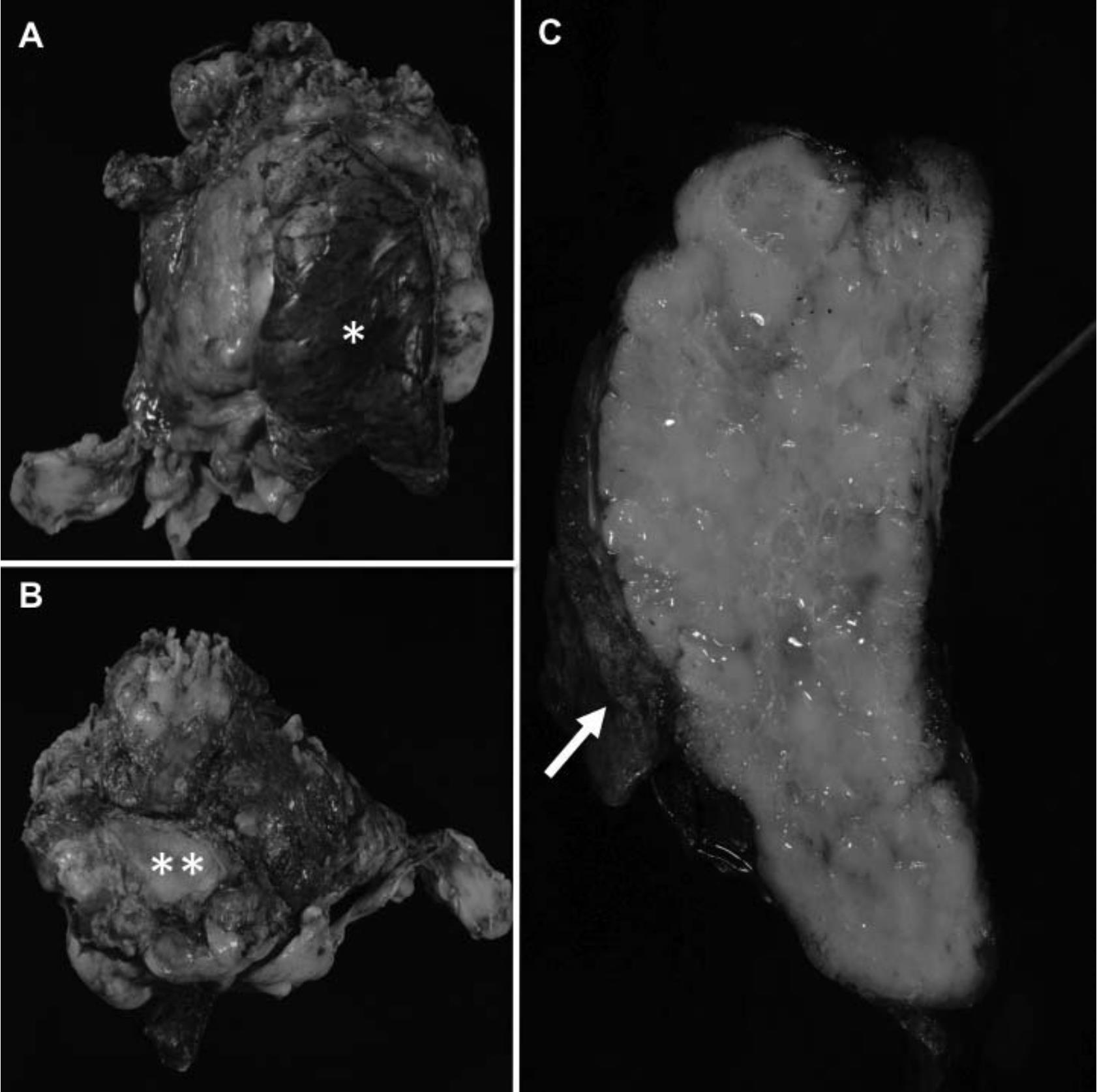
Figure 2 A grossing process of thymoma resection specimens. The first step is specimen orientation and identification of any important attached structures such as lung tissue (A*) and pericardium (B**). Once all the resection margins and important attached structures are well oriented, ink these key areas and serially section the specimen from superior to inferior in a bread loafing manner. Then inspect characteristics of the tumor, integrity of its capsule and its relationship with attached tissues/ important structures and resection margins. As shown in (C), invasion of the tumor into the attached lung tissue (arrow) and pericardium (pointed with a stylus) is grossly noted.
Gross description of resection specimens of thymomas should include:13
1. Specimen:
- a. Weight (g) and size (cm) in 3 dimensions
- b. Anatomic orientation (if any) provided by a surgeon
- c. Condition of the capsule (intact or ruptured)
- d. Attached important structure(s)
2. Tumor:
- a. Size (cm) in 3 dimensions (listed the largest dimension first)
- b. Description of the tumor including color, texture, nodularity, and presence of necrosis and/or fibrous bands
- c. Documentation of gross invasion or relationship of the tumor to capsule, attached tissue or adherent structures, and surgical resection margins
3. Description of non-neoplastic thymus and/or attached tissue
4. Lymph node(s):
- a. Number of lymph node received
- b. Size (cm) in greatest dimension
- c. Description of cut surfaces
5. Clear documentation of each sampled block with a section code indicating a location within the tumor/ specimen where the sampled tissue was taken
Block Selection
Capsular status and tumor invasiveness are the most important prognostic parameters. Thus, careful inspection of tumor circumference is very essential. Tissues submitted for microscopic examination should include representative tumor areas, which illustrate the interface between the tumor and uninvolved thymic tissue, relationship of the tumor to a capsule and important adherent structures, and intratumoral heterogeneity; and any areas, which are suspicious for margin involvement. Additional blocks may be required for non-neoplastic thymic tissue and areas with other pathological findings. Because thymomas are microscopically heterogeneous, adequate tissue sampling for microscopic examination is of paramount importance for correct diagnosis and subtyping. If a tumor is not more than 5 cm in greatest dimension, the tumor area must be submitted entirely for microscopic examination in at least 5 blocks. For a tumor that is greater than 5 cm in greatest dimension, submission at least one block of tissue for each centimeter of greatest tumor dimension is suggested. In case that the tumor is cystic, solid tissue at the periphery of the cyst must be extensively sampled to obtain diagnostic tissue for exclusion of other possible cystic tumors of the thymic region such as germ cell tumors, metastatic carcinomas, and malignant lymphomas.132425
Partially encapsulated or unencapsulated thymomas with tumor lobules juxtaposing to surrounding thymic tissue or mediastinal fat can pose a problem in evaluation of tumor invasiveness, especially when modified Masaoka-Koga classification or Moran staging system being applied. In this particular situation, demonstration of nonneoplastic thymic or fatty tissue being surrounded by the tumor lobules is indicative of microscopic tumor invasion into thymic or surrounding fatty tissue and represents stage IIb/IV and I/III tumors in modified Masaoka-Koga classification and Moran staging system, respectively. Therefore, thorough sampling of the interface between tumor nodules and adjacent non-neoplastic thymic/fatty tissue is necessary for demonstration of the aforementioned feature. However, this issue might not be problematic if the forthcoming (8th) edition of the TNM classification is used, as presence of tumor invasion into adjacent non-neoplastic thymic/fatty tissue without involvement of other mediastinal structure does not upstage the tumor from stage I to stage II in this staging system. Despite the controversy regarding a role of microscopic tumor invasion into thymic or surrounding fatty tissue in prognostic prediction, we strongly recommend that thorough sampling of tumor borders should be done in any thymomas, regardless of the presence of capsule, as it is important in documentation of tumor extent in the pathology report.
Block selection in grossing thymoma resection specimens is illustrated in Figure 3.

Figure 3 Block selection in grossing thymoma resection specimens. Tissue submitted for microscopic examination should include (A) an interface between a tumor and attached important structures (shown in this figure are lung tissue and pericardium), (B) an interface between a tumor and non-neoplastic thymic tissue/mediastinal fat, (C) areas which are suspicious for margin involvement, and (D) areas representing intratumoral heterogeneity.
HISTOLOGICAL CLASSIFICATION
Tumor histological classification systems are usually devised to facilitate communication between pathologists and clinicians as well as to show a correlation between the different categories and patient outcome or tumor biology. In a realm of thymic epithelial neoplasms, two important histological classifications have been claimed to fulfill the aforementioned purposes of tumor classification system: the World Health Organization (WHO) classification and Suster-Moran classification.
World Health Organization (WHO) Classification
In 1999, the WHO Committee for the International Histological Classification of Tumors had first proposed a histologic classification of thymic epithelial neoplasms that was based on a combination of letters and numbers. Principally it recognized 2 basic types of thymoma - those comprising oval or spindle neoplastic cells (type A), and those comprising round or epithelioid neoplastic cells (type B). Type B was further subdivided into B1, B2 and B3, based on a decreased number of lymphocytes and increased cytologic atypia of the epithelial cells. Type AB represented an admixture of type A and B, and type C referred to tumors showing overt cytologic features of malignancy. In the subsequent 2004
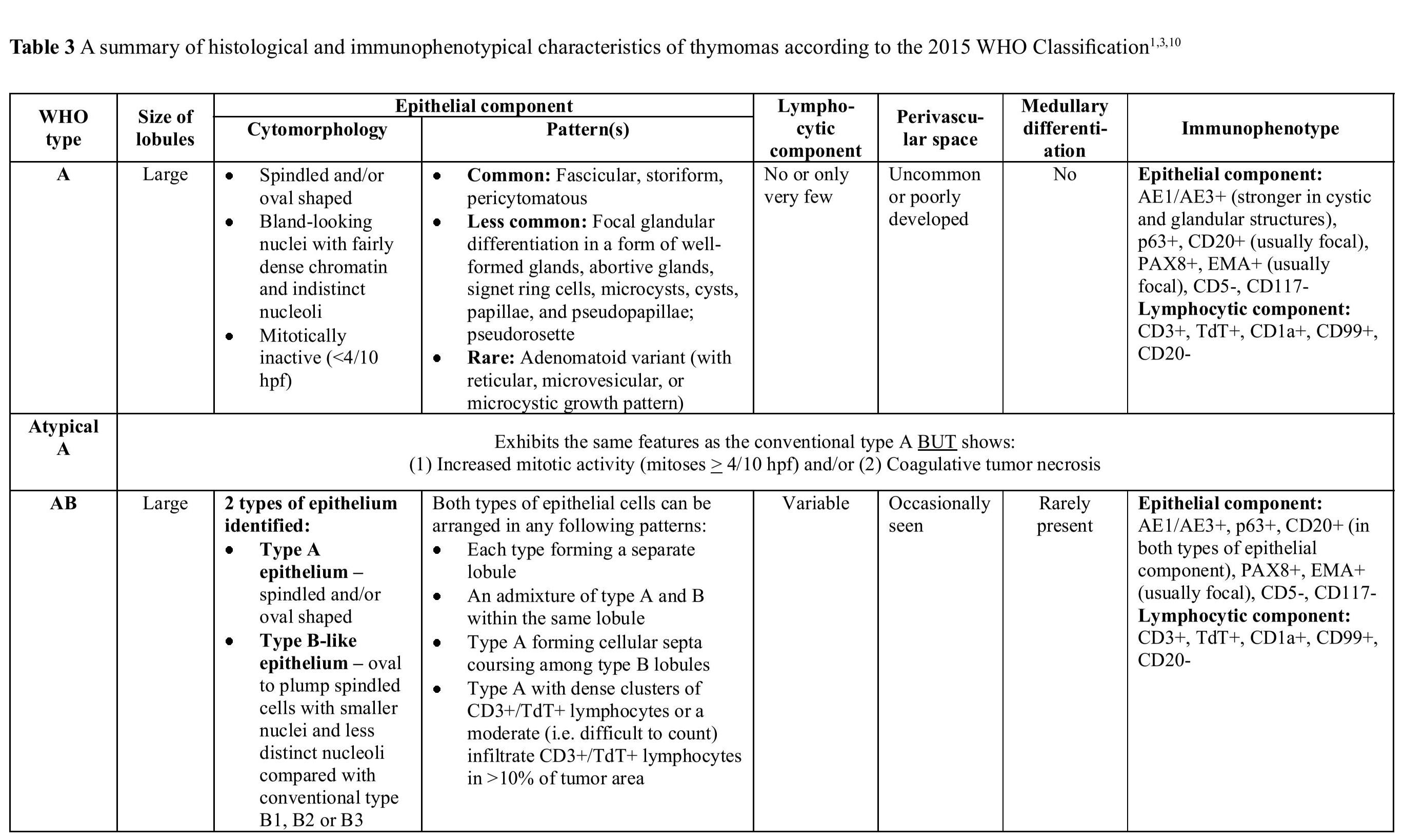
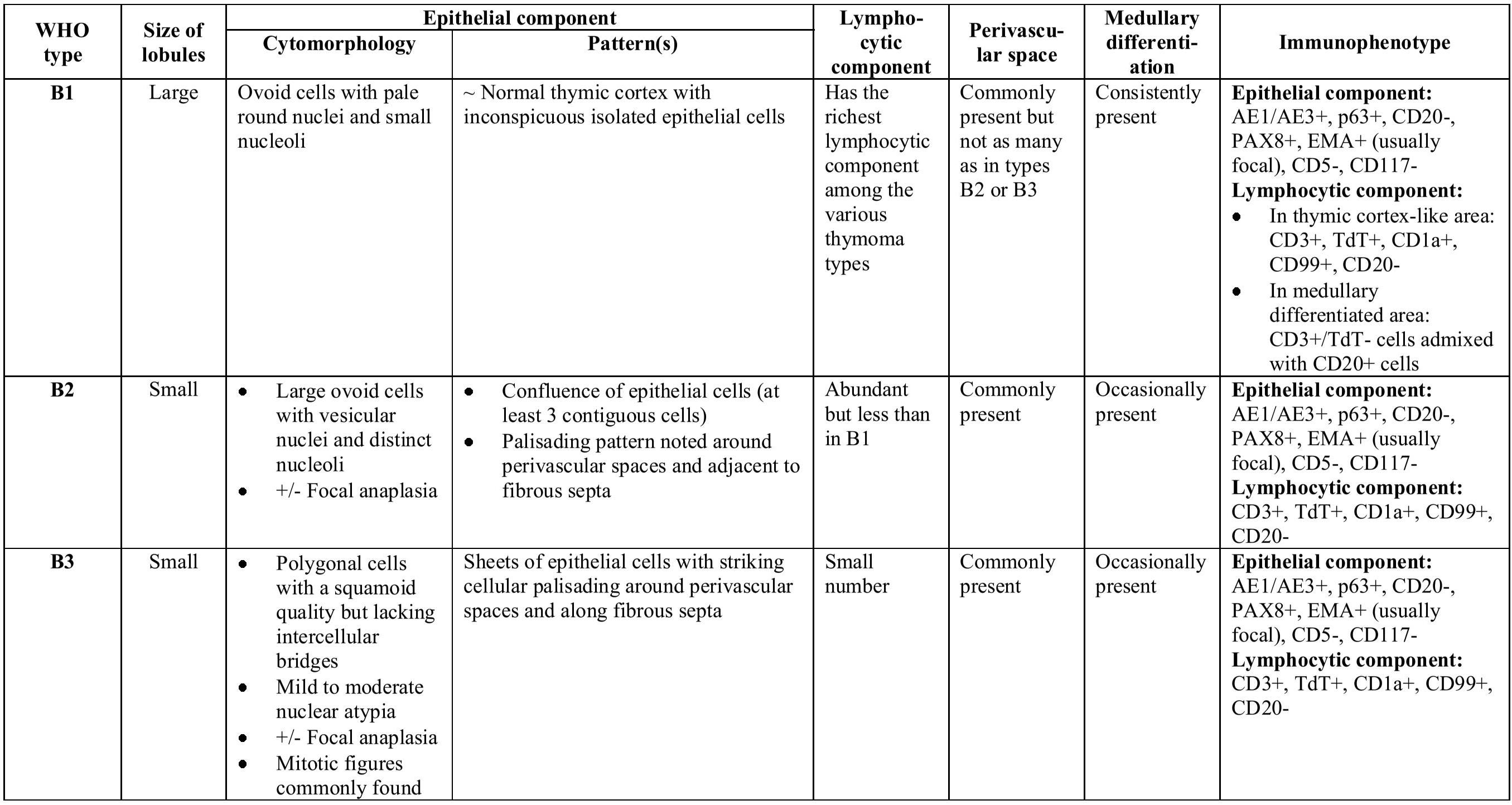
WHO Classification of thymic epithelial neoplasms, the same terminology was retained for the types A, AB, and B1 through B3; however, type C thymoma was placed in a separate category designated thymic carcinoma. In addition, a few unusual morphologic types were added, such as metaplastic thymoma and micronodular thymoma with lymphoid stroma, among others.26 The most recent (2015) WHO Classification of thymic epithelial neoplasms has made a few minor modifications hoping to improve interobserver reproducibility. These changes include: (1) refinement of diagnostic criteria for each thymoma type; (2) introducing a new concept of “atypical type A thymoma”; and (3) proposal of a new rule for reporting thymic epithelial neoplasms with heterogeneous histological features.310 Histological and immunophenotypical characteristics of major WHO thymoma types are summarized in Table 3, Figure 4 and Figure 5.
In a thymic epithelial neoplasm with more than one component, different components should be listed and quantified with 10% increments beginning with the predominant histology. Thymoma components of < 10% can be disregarded; however, if a carcinoma component is present, such tumors should be labeled as carcinomas with listing of the proportion, differentiation and grade, followed by the list of thymoma component(s) as mentioned above.310
Table 3 A summary of histological and immunophenotypical characteristics of thymomas according to the 2015 WHO Classification1310
Suster-Moran Classification
Suster, et al27 had proposed a novel classification system for thymic epithelial neoplasms in 1999, claiming that their newly proposed system represented a simplified approach to the histological classification of these tumors. A summary of characteristic features of categories in this classification system is shown in Table 4, Figure 4 and Figure 5.
Table 4 Characteristic features of categories in Suster-Moran classification27
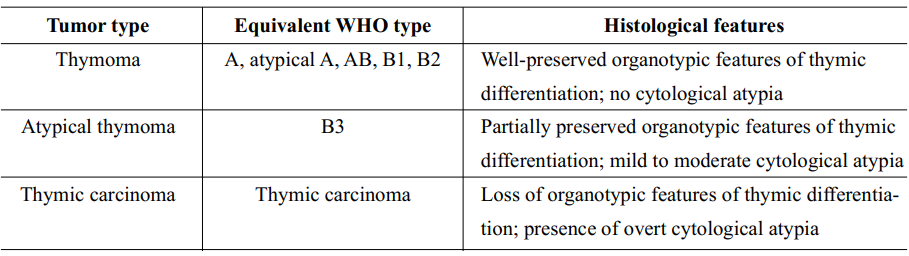
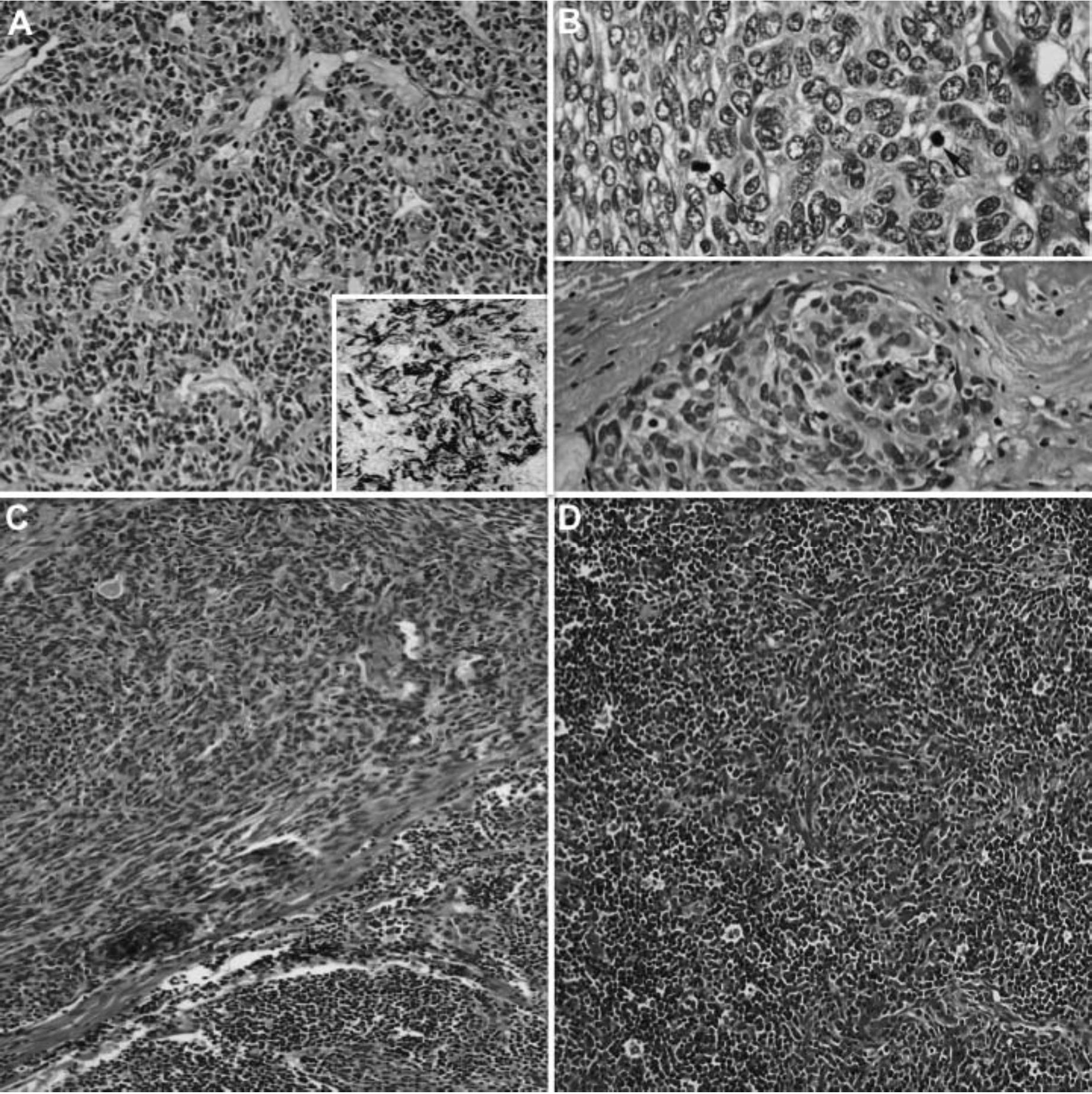
Figure 4 Characteristic histological features of thymomas with spindle-shaped thymic epithelial cells. (A) WHO type A with CD20 reactivity (inset), (B) atypical WHO type A with increased mitotic figures > 4/10 hpf (arrows) and/or coagulative tumor necrosis, and (C and D) WHO type AB with a blend or combination of lymphocyte-rich and spindle cell areas. All of these thymomas are equivalent to a “thymoma” category in Suster-Moran Classification
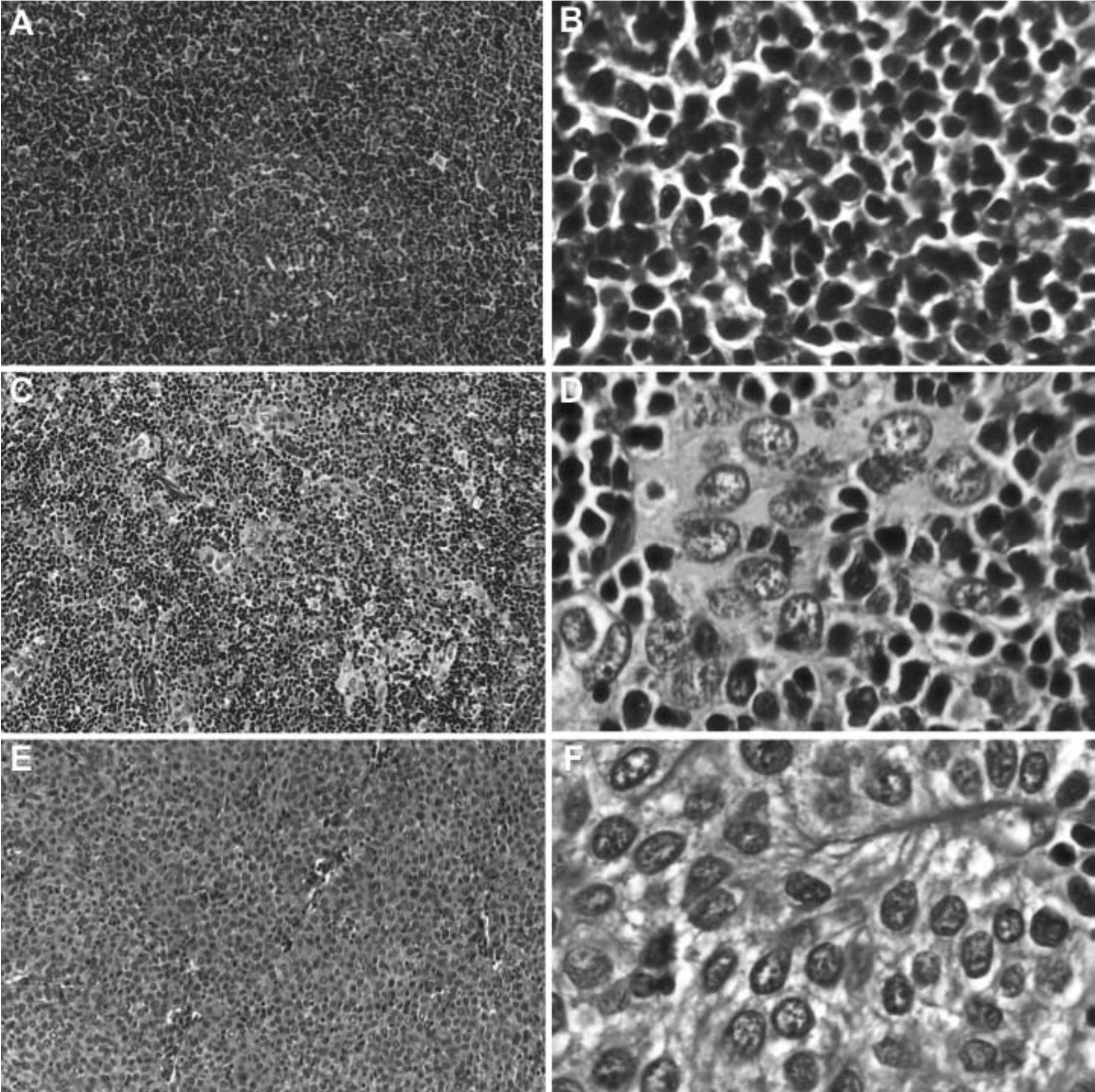
Figure 5 Characteristic histological features of thymomas with round or polygonal-shaped thymic epithelial cells. (A and B) WHO type B1 with predominantly small lymphocytes and inconspicuous neoplastic epithelial cells, (C and D) WHO type B2 with ratio of small lymphocytes and neoplastic epithelial cells about 1:1, and (E and F) WHO type B3 with sheeting of neoplastic epithelial cells and scant lymphocytes. According to Suster-Moran Classification, WHO types B1 and B2 are considered to be in a “thymoma” category whereas WHO type B3 is equivalent to “atypical thymoma”.
Reproducibility and prognostic prediction
Regarding reproducibility, WHO Classification has suffered a lot of criticisms on being too complicated and impractical for general pathologists, which results in poorer reproducibility comparing to Suster-Moran Classification.142328-30
The role of WHO Classification of thymomas as a prognostic predictor is controversial. Some studies have shown that the WHO classification is an independent prognostic factor with 10-year disease-free survival rates decreasing from A, AB, B1, B2 and B3, respectively,31-35 whereas others have demonstrated that type B3 tumors have a worse prognosis and suggested that grouping thymic epithelial neoplasms into 3 subtypes (A, AB, B1, B2 vs. B3 vs. thymic carcinoma) is more predictive of survival as there is no statistically significant difference in the prognosis of tumor types A and b.H28’2936-39 In contrast, a good correlation between histological types and clinical outcome has been consistently established when using Suster-Moran Classification despite not being an independent prognostic predic-tor.23-29
REPORTING GUIDELINE
Core biopsy specimen
In spite of the importance of specimen adequacy in histopathological evaluation of biopsy specimens, there are no evidence-based guidelines for specimen adequacy evaluation in mediastinum biopsy. A panel of experts has proposed that “at least 3 representative biopsy cores using a 19-gauge needle or larger” should be considered as a minimum criteria for specimen adequacy in mediastinum biopsy.40 However, in our daily practice, fewer numbers of biopsy cores are considered adequate for evaluation if the obtained tissues are well preserved and truly represent the tumor or lesion of interest.
If a thymic epithelial neoplasm with typical histological features of thymomas is identified, specific diagnosis as “thymoma” can be rendered even in core biopsy specimens. Nevertheless, specific subtyping of the tumor should be based on thorough examination of a resection specimen regarding heterogeneous nature of thymomas. Inclusion of information in a “Comment” section providing a brief discussion of the potential limitations and pitfalls of a particular diagnosis and a need for correlation between clinical, radiological and histopathological findings in making diagnosis is also suggested. In problematic cases, immunohis-tochemistry is useful for distinguishing thymomas from other tumors arising in mediastinum.
Resection specimen
Once a diagnosis of thymoma has been made in a resection specimen, the surgical pathology report should include (1) histological type (according to WHO Classification or Suster-Moran Classification); (2) greatest dimension of the tumor; (3) capsular integrity and invasion - localized (i.e. encapsulated though capsule may be partially absent), minimally invasive (i.e. tumor penetrates through capsule into adjacent fat < 3 mm), or invasive (i.e. tumor penetrates through capsule into adjacent fat > 3 mm or infiltrates into surrounding structures); (4) margin status (distance in mm reported whenever < 3 mm); (5) pathology of remaining thymus; and (6) regional lymph node status.1324
CONCLUSION
Thymoma is a thymic epithelial neoplasm that demonstrates some organotypic features of normal thymus and is accompanied by variable numbers of reactive lymphoid cells. Nowadays it is considered an epithelial neoplasm of at least low malignant potential and should be handled properly as a malignant neoplasm. Clinical outcomes mainly depend on tumor invasiveness, which is represented by clinical staging, and status of resectability. Although there are controversies regarding a correlation between histological features and prognosis, it has been shown that histological classification of thymomas might play some roles in predicting survival and prognosis.
REFERENCES
1. Chan JKC. Tumors of the lymphoreticular system, including spleen and thymus: Part C The Thymus. In: Fletcher CDM, editor. Diagnostic Histopathology of Tumors. Philadelphia: Elsevier Saunders; 2013. p. 1558-602.
2. Suster S, Moran CA. Mediastinum. In: Weidner N, Cote RJ, Suster S, Weiss LM, editors. Modern Surgical Pathology. Philadelphia: Saunders Elsevier; 2009. p. 454-516.
3. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG (eds.): WHO Classification of Tumours of Lung, Pleura, Thymus and Heart (4th ed). IARC: Lyon 2015.
4. Weissferdt A, Moran CA. Immunohistochem-istry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014;22(7):479-87.
5. Adam P, Hakroush S, Hofmann I, Reidenbach S, Marx A, Strobel P. Thymoma with loss of keratin expression (and giant cells): a potential diagnostic pitfall. Virchows Arch 2014;465(3):313-20.
6. Ordonez NG. Value of PAX 8 immunostaining in tumor diagnosis: a review and update. Adv Anat Pathol 2012;19(3):140-51.
7. Weissferdt A, Moran CA. Pax8 expression in thymic epithelial neoplasms: an immu-nohistochemical analysis. Am J Surg Pathol 2011 ;35(9): 1305—10.
8. Dotto J, Pelosi G, Rosai J. Expression of p63 in thymomas and normal thymus. Am J Clin Pathol 2007;127(3):415-20.
9. Wu M, Sun K, Gil J, Gan L, Burstein DE. Im-munohistochemical detection of p63 and XIAP in thymic hyperplasia and thymomas. Am J Clin Pathol 2009;131(5):689-93.
10. Marx A, Strobel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9(5):596-611.
11. Pan CC, Chen WY, Chiang H. Spindle cell and mixed spindle/lymphocytic thymomas: an integrated clinicopathologic and immunohisto-chemical study of 81 cases. Am J Surg Pathol 2001;25(1): 111-20.
12. Weissferdt A, Hernandez JC, Kalhor N, Moran CA. Spindle cell thymomas: an immunohisto-chemical study of 30 cases. Appl Immunohis-tochem Mol Morphol 2011;19(4):329-35.
13. Weydert JA, De Young BR, Leslie KO, Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of surgically resected thymic epithelial tumors. Am J Clin Pathol 2009;132(1):10-5.
14. Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol 2015;10(4):691-700.
15. Suster S, Moran CA. Problems in the classification of thymoma. Diagn Histopathol 2010;16:221-7.
16. Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6(7 Suppl 3):S1710-1716.
17. Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44(5):359-67.
18. Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9(9 Suppl 2):S65-72.
19. Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9(9 Suppl 2):S81-87.
20. Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9(9 Suppl 2):S73-80.
21. Moran CA, Walsh G, Suster S, Kaiser L. Thymomas II: a clinicopathologic correlation of 250 cases with a proposed staging system with emphasis on pathologic assessment. Am J Clin Pathol 2012;137(3):451-61.
22. Weissferdt A, Moran CA. Staging of thymic epithelial neoplasms: thymoma and thymic carcinoma. Pathol Res Pract 2015;211(1):2-11.
23. Roden AC, Yi ES, Jenkins SM, et al. Reproducibility of 3 histologic classifications and 3 staging systems for thymic epithelial neoplasms and its effect on prognosis. Am J Surg Pathol 2015;39(4):427-41.
24. Detterbeck FC, Moran C, Huang J, et al. Which way is up? Policies and procedures for surgeons and pathologists regarding resection specimens of thymic malignancy. J Thorac Oncol 2011 ;6(7 Suppl 3):S1730-1738.
25. Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms. Impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol 2000;114(5):760-6.
26. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds.): WHO Classification of Tumours of Lung, Pleura, Thymus and Heart (3rd ed). IARC: Lyon 2004.
27. Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma. A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol 1999; 111(6): 826-33.
28. Rieker RJ, Hoegel J, Morresi-Hauf A, et al. Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J Cancer 2002;98(6):900-6.
29. Rossi G, Costantini M, Tagliavini E, Barbieri F,
Migaldi M, Casali C. Thymoma classification: does it matter? Histopathology 2008;53(4):483-4.
30. Verghese ET, den Bakker MA, Campbell A, et al. Interobserver variation in the classification of thymic tumours--a multicentre study using the WHO classification system. Histopathology 2008;53(2):218-23.
31. Chen G, Marx A, Chen W-H, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95(2):420-9.
32. Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94(3):624-32.
33. Park MS, Chung KY, Kim KD, et al. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg 2004;78(3):992-997; discussion 997-998.
34. Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer 2005;50(1):59-66.
35. Strobel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22(8):1501-9.
36. Kim DJ, Yang WI, Choi SS, Kim KD, Chung KY. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinico-pathologic study of 108 patients and literature review. Chest 2005;127(3):755-61.
37. Marchevsky AM, Gupta R, McKenna RJ, et al.
Evidence-based pathology and the pathologic evaluation of thymomas: the World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer 2008;112(12):2780-8.
38. Sonobe S, Miyamoto H, Izumi H, et al. Clinical usefulness of the WHO histological classification of thymoma. Ann Thorac Cardiovasc Surg 2005;11(6):367-73.
39. Moran CA, Weissferdt A, Kalhor N, et al. Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. Am J Clin Pathol 2012;137(3):444-50.
40. Marchevsky A, Marx A, Strobel P, et al. Policies and reporting guidelines for small biopsy specimens of mediastinal masses. J Thorac Oncol 2011;6(7 Suppl 3):S1724-1729.


