Comparison of triple-dose single-donor platelet collection by the Amicus™ and the Trima Accel® blood-cell separators.
Woraprapa Apaikawee1, Viroje Chongkolwatana1,
Sasijit Vejbaeya1, Thanaphak Warindpong1
1. Department of Transfusion Medicine, Faculty of Medicine Siriraj Hospital, Bangkok, Thailand
Corresponding author: Viroje Chongkolwatana
Department of Transfusion Medicine, Faculty of Medicine Siriraj Hospital,
Wanglang Road, Bangkoknoi, Bangkok, Thailand 10700
Tel: 662-419-7598 Fax: 662-412-8419 Email:Viroje.cho@mahidol.ac.th
Received 17th December 2015; Accepted 2nd February 2016
ABSTRACT
Objective: The purpose of this study was to compare triple-dose single-donor platelet collection using Amicus™ and Trima Accel® blood-cell separators.
Method: The same 25 donors underwent triple-dose single-donor platelet collection using the Amicus™ and Trima Accel® systems. The target endpoint was > 9.5 x 1011 platelets. Processing time, apheresis characteristics, donor safety, and satisfaction, were evaluated. In all, 18 donors completed the study; all of the resulting 36 donations were evaluated.
Results: The collection efficiency of both systems were not statistically different (80.25±10.64 vs. 76.63±6.06 %, p=0.228), the amount of whole blood volume processed was high (5274.50±874.86 vs. 4428.28±593.09 ml, p <0.001), and it used more infused ACD-A (593.56 ±92.74 vs. 521.17± 72.02 ml, p=0.010). The Trima Accel® process was faster (101.10±16.58 vs. 88.61±16.71 min, p=0.002). Most donors expressed satisfaction. The Trima Accel® system scored higher because its comparative speed (9.17 vs.9.22, p=0.805). After separation into blood constituents, all products had < 5.0 x106 (1.04±3.3 vs. 0.44 ±1.05 x 106/unit, p=0.372) residual white blood cells. No severe adverse reactions were noted with either of the instruments.
Conclusion: Our experimental data showed that triple-dose platelet collection was safe using either system. The platelet yields satisfied American Association of Blood Banks (AABB) guidelines.
Keywords: Triple-dose single-donor platelet, Amicus™, Trima Accel®, blood cell separators, comparison
INTRODUCTION
Platelets are transfused to treat bleeding in situations where clinically circulating platelets are decreased, or platelets are functionally abnormal. We use platelets for bleeding prophylaxis and treatment at low platelet. The demands for plateletpheresis has been steadily increasing1. In 2013, the Thai Red Cross Society produced a total of 12,532 plateletpheresis units2. Plateletpheresis production has been increasing steadily over the past 10 years, while the recruitment and retention of voluntary and eligible blood donors has become increasingly difficult. High-dose plateletpheresis enables the collection of multiple units from the same donor in a single donation, and may potentially help resolve the problem by reducing the costs and risks inherent with allergic transfusion34. Recently, technologies have been developed to increase platelet yield, resulting in the collection of multiple platelet units from one donor1. The latest generation of apheresis machines i.e. the Amicus™ and the Trima Accel® have shown significant progre ss in collection efficiency, citrate management, and donor comfort. These systems are specifically designed to harvest at least two units of platelet (PLT) components, or even more and different blood products4. In addition, product quality, particularly in terms of leukoreduction, should be consistent5. This study aimed to compare different automated blood collection systems for triple-dose singledonor platelet collection for feasibility and safety of triple-dose collection, and donor satisfaction.
MATERIALS AND METHODS
Donors
Donors were required to meet the AABB criteria6 for whole blood donation, and repeated donors met the inclusion criteria at the Department of Transfusion Medicine, Faculty of Medicine, Siriraj Hospital. All donors gave written informed consent, as ethical approved by the Siriraj Institutional Review Board with ethic code 530/2555(EC2) , before inclusion into the study. The study ran from October 2012 to July 2013. Twenty-five donors were eligible for triple-dose platelet collection. The final donation was at least 4 weeks after prior donations. The pre-procedure platelet count was > 300 x 103 / μL, while the postdonation platelet count was not < 100 x 103/ μL
Plateletpheresis procedures
Twenty-five healthy blood donors5 were enrolled into the study. Blood samples were collected from each donor and tested using a standard blood-borne pathogen test panel (HIV-Ag, Anti-HIV, HBsAg, Anti-HCV, etc.). For the collection of triple-dose single-donor platelets, donors were randomized by simple random sampling for the first instrument. At least four weeks later, each donor was transferred to the second blood-cell separator. Plateletpheresis collections were performed using the Trima Accel® Version 5.1 software and Amicus™ Version 3.21 software.
All pre-procedure, post-procedure and product platelet counts were performed with the same automated cell counter (Sysmex XS-800i). Post-procedure samples were drawn from the access site after the end of reinfusion with 7-10 mL waste sample removal. After collection, the platelet products were gently agitated in an incubator at 20-24° C overnight. White blood cell (WBC) counts were performed manually with a modified Nageotte chamber7. After each donation, the donors completed the questionnaire. We used the following formulas for calculation.
Product volume (mL) is calculated by weight of platelets, divided by specific gravity.
Volume = Net weight (g) / 1.030 (g/mL)
Platelet yields and collection efficiency.
Platelet yield = Product volume (mL) x product count (platelet / L) x conversion factor (1,000 L/ mL)
Collection efficiency = (platelet yield / total platelets processed#) x 100
Residual white blood cells
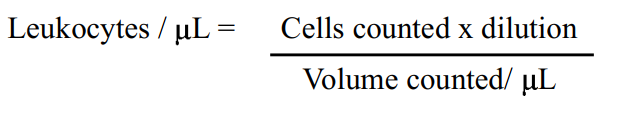
Statistical analysis
All data were recorded and analyzed using SPSS for Windows (version 13)89. The results were presented as mean (X) ± standard deviation (SD). Numerical data were tested for normal distribution with the Kolmogorov-Smirnov test. Then, the normal distribution was compared with the t-test for paired samples, or the non-normal distribution compared with the Wilcoxon signed-rank test. Ordinal data were compared using the Wilcoxon signed-rank test. Nominal data were compared the McNemar test; a P-value (p) < 0.05 was considered statistically significantly different.
RESULTS
Twenty-five donors were included into this study. Seven donors were unavailable for the second donation and could not be evaluated. In all, 18 donors completed the study and all 36 resulting donations were evaluated. Donor characteristic data are summarized in Table 1. The same donors donated twice, so there were no differences between the two groups for sex, age, weight, height, and total blood volume.
Comparative efficiency of automated plateletpher-esis collection system
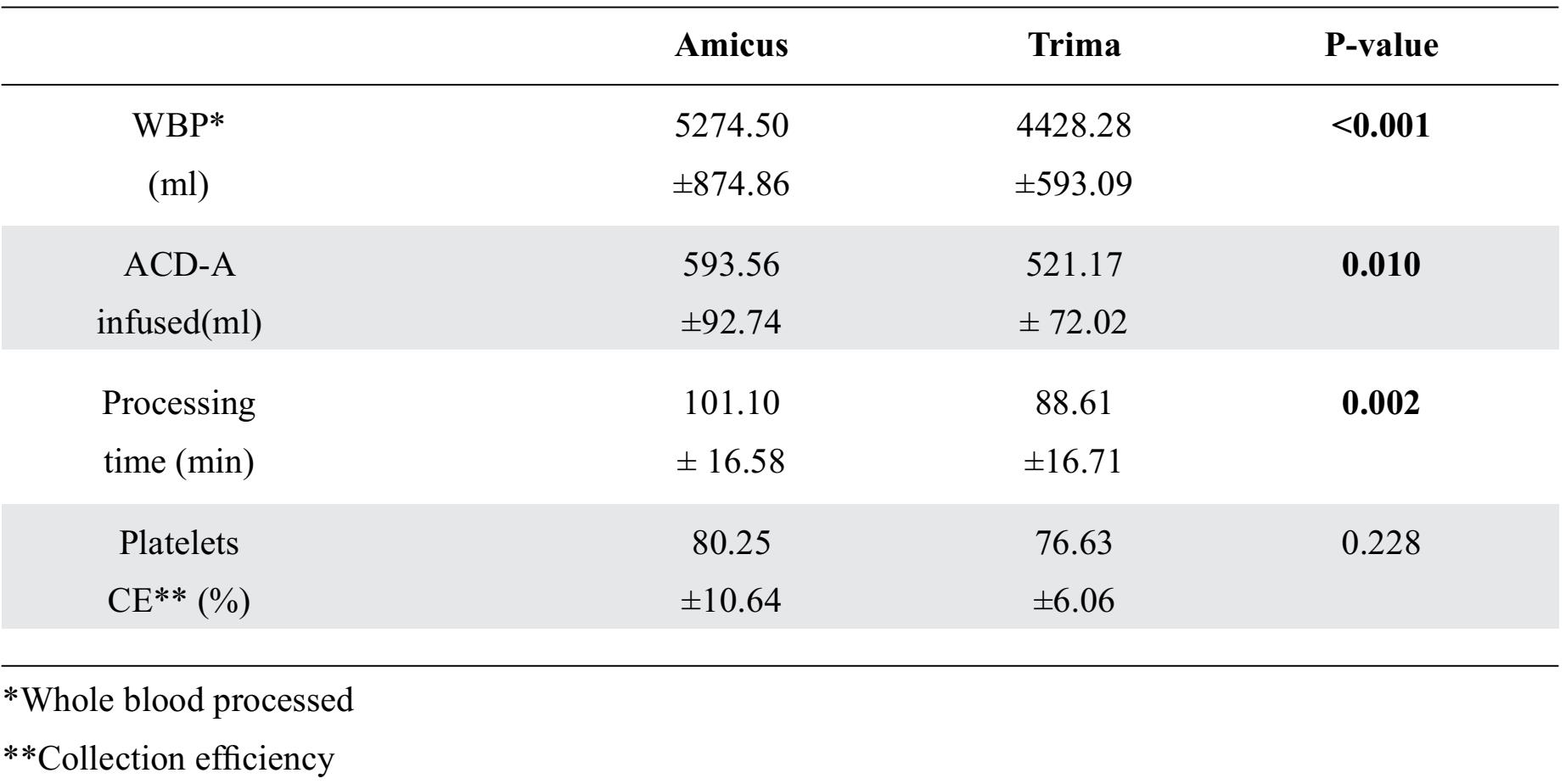
The apheresis procedure shown in Table 2 that the platelet collection efficiency of the Amicus™ and the Trima Accel®, respectively (80.25±10.64 vs. 76.63±6.06 °%) was not statistically significantly different. For whole blood processed, ACD-A, and processing time, the Amicus™ used significantly more whole blood and time than the Trima Accel®.
Table 1. Characteristics data of donors
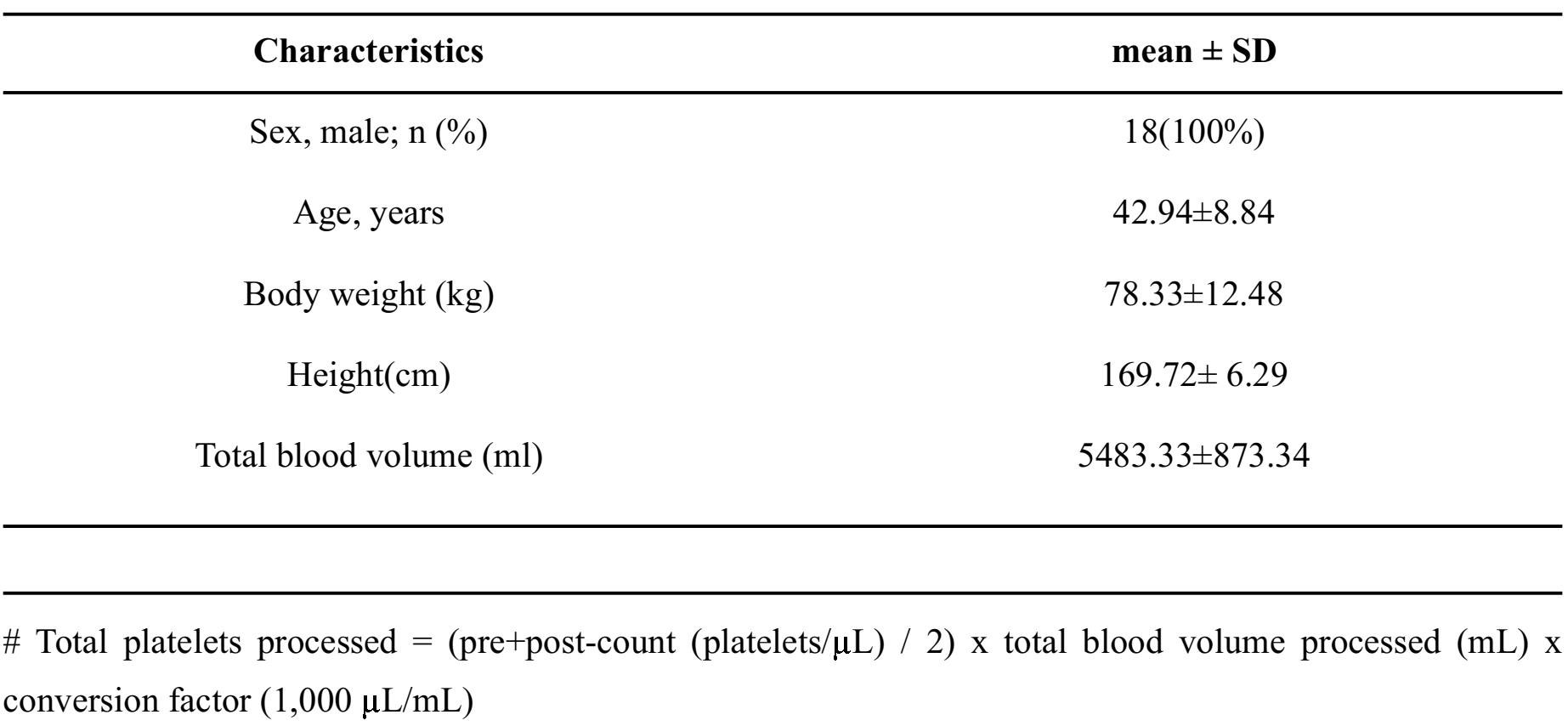
Table 2. Apheresis procedure characteristic expressed as mean ± SD
Comparison of triple-dose single-donor platelet-pheresis product
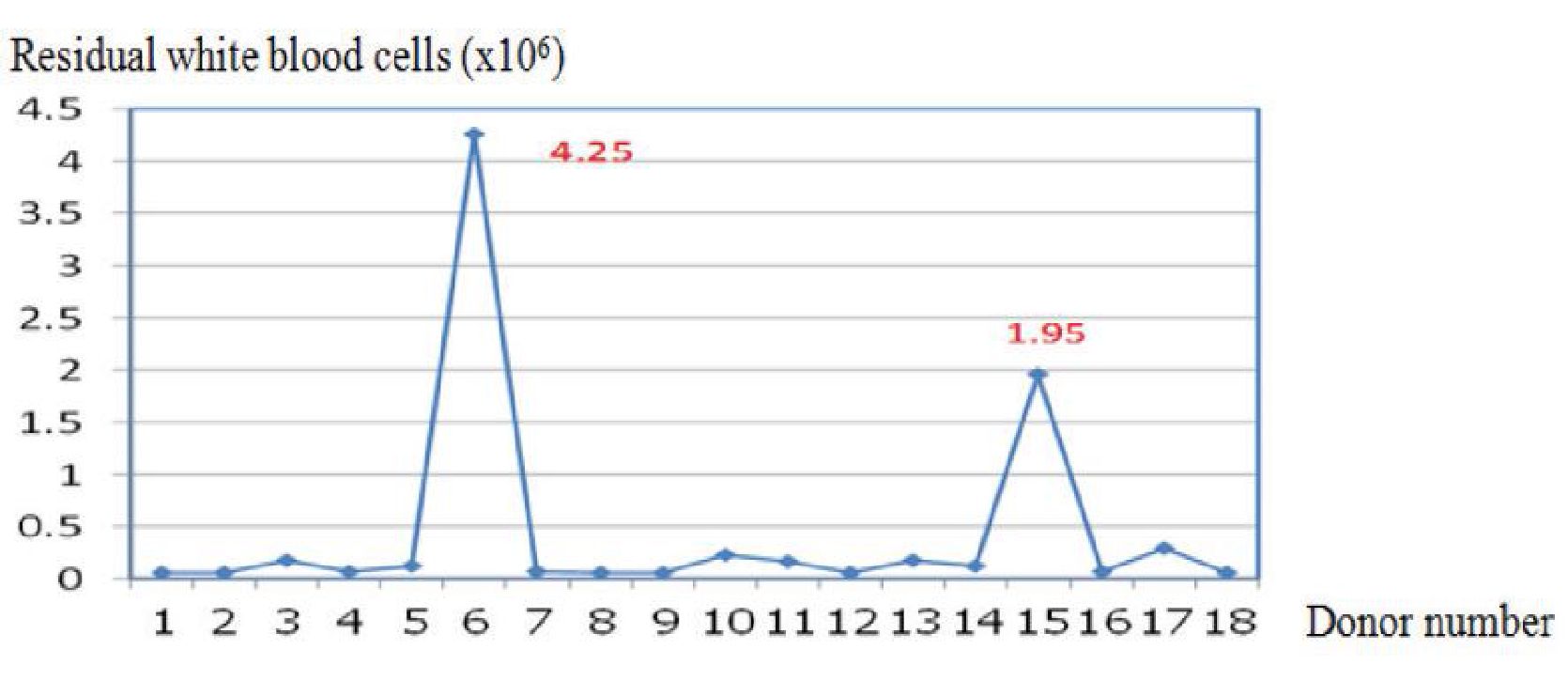
Platelet production is shown in Table 3. The two automated blood collectors showed statistically significant differences in platelet volume and platelet yield. Overall, the Amicus™ had significantly greater characteristics than the Trima Accel®. However, the residual WBC (1.04±3.3 vs. 0.44±1 x 106 /unit) levels were not statistically significantly different. Figures 1 and 2 show the residual white blood cells measured from 36 donations produced with the Amicus™ and the Trima Accel®. All products before separation from both instruments had < 1x106 residual white blood cells counts10, except for 4 collections (two from the Amicus™ and two from the Trima Accel®). One Amicus™ procedure (2.63%) showed a count > 5x106, whereas three (7.89%) showed a count > 1x106, one of the three from the Amicus™ procedure, and one case showed > 5x106 (14.4x106) but after filtration, it was < 1x106 (0.06 x106).
Table 3. Productivity expressed as mean ± SD
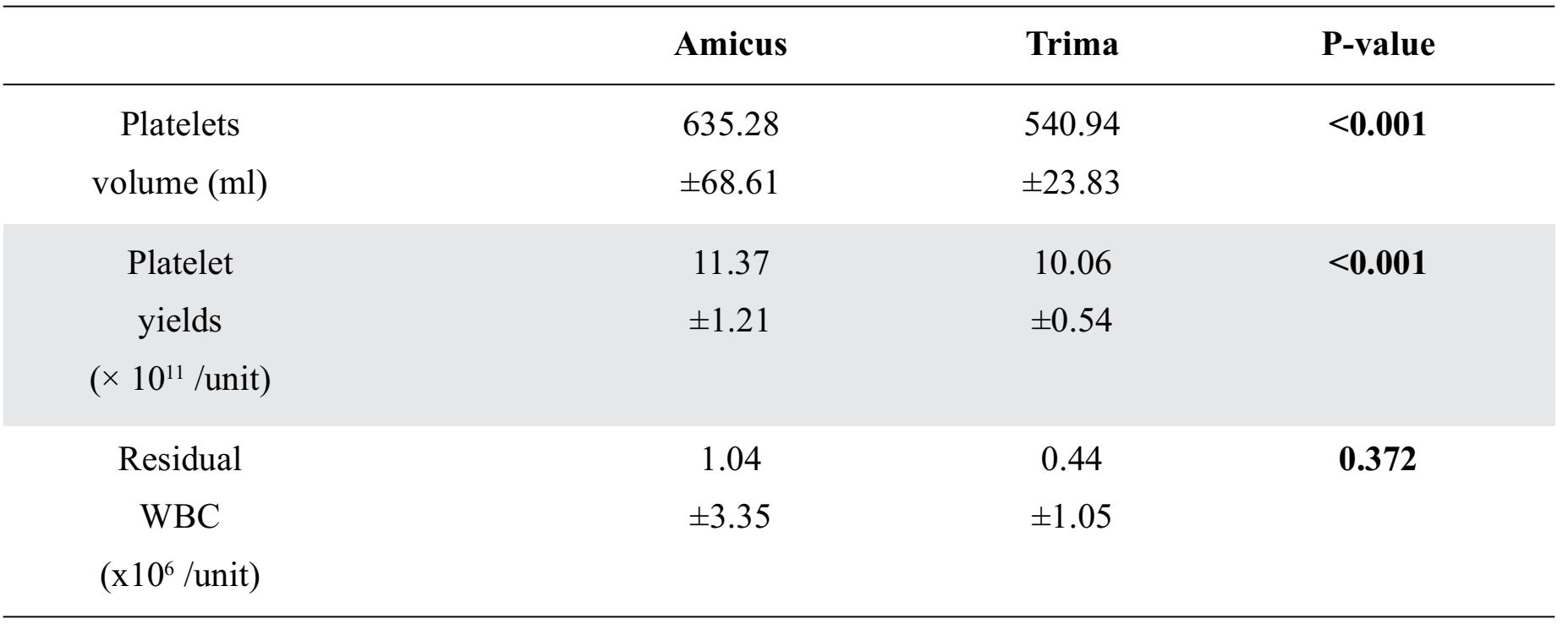
Figure 1 The Residual white blood cells (x106) of the Amicus™
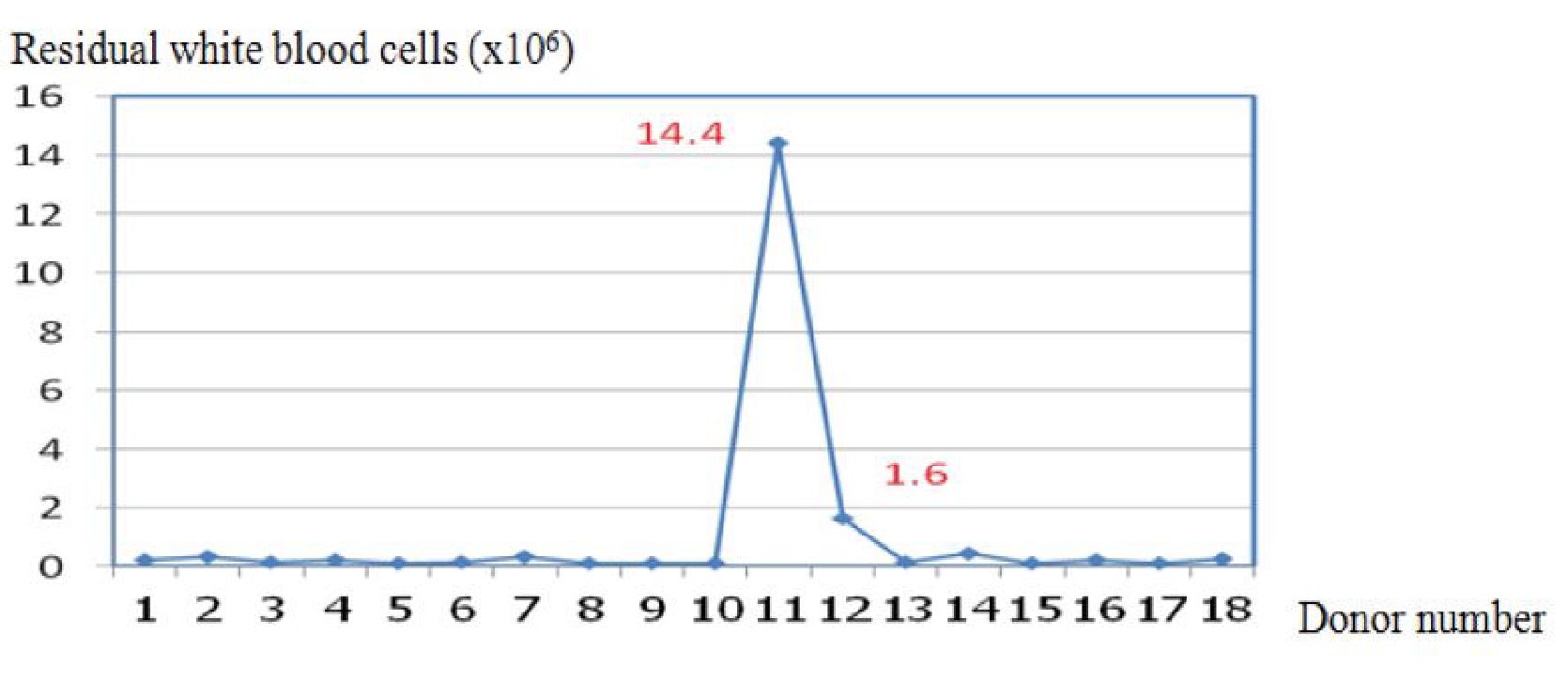
Figure 2 Residual white blood cells (x106) of the Trima Accel®.
Donor safety and donor satisfaction
Donor safety expressed by donor adverse reactions are summarized in Table 4. Both instruments had no serious adverse reactions. However, all mild adverse reactions occurred and were not significantly differences between the Amicus™ and the Trima Accel® .The mild citrate adverse reactions (gradel) occurred in both instruments. Therefore, the treatment was relatively simple by giving oral calcium supplementation to donors. When donation process finished, donors answered a questionnaire. All of donor wanted to donate platelet again with the Trima Accel® more than the Amicus. Donors prefered the Trima Accel® due to a shorter duration of donation process. While donors who choose the Amicus™ gave the main reason with smooth draw and return flow.
Table 4. Donor adverse reactions expressed as n (percentages)
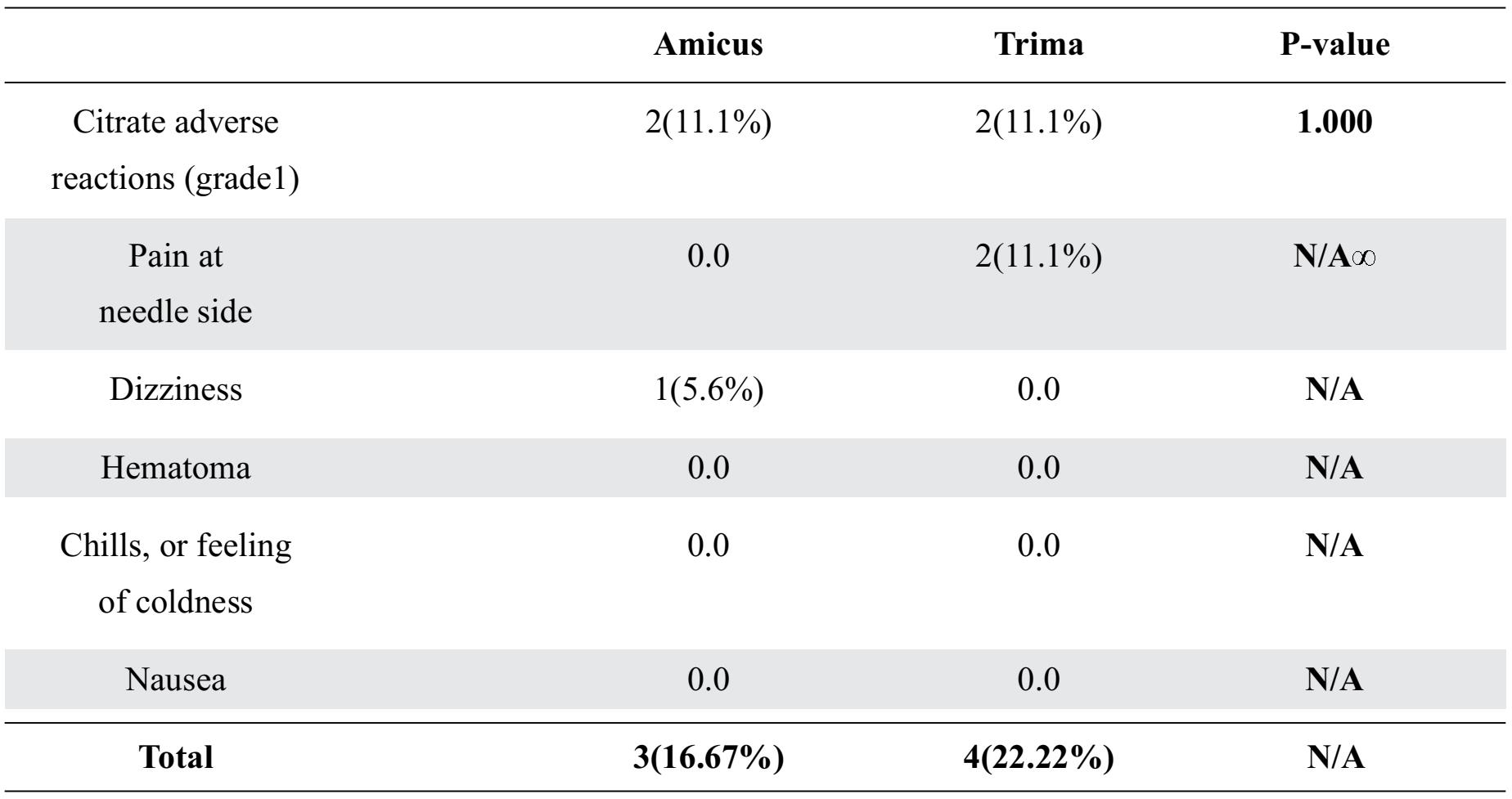
*Not available for analysis
DISCUSSION
High dose plateletpheresis collection can contribute to providing sufficient blood supply under a situation of limited human resources and shrinking donor population. The aim of this study was to compare apheresis equipment with respect to productivity, donor safety and donor satisfaction, respectively11.
We evaluated the differences between the Amicus™ and Trima Accel® systems with each donor. Because donations were collected from a random device order per donor, sample biases between the two devices were minimized. Efficient plateletpheresis procedures are defined not only by platelet product characteristics, but also pre- and post-procedure donor cells counts, processed blood volume, and the use of ACD-A. Post-procedure, donor-cell counts were lower than pre-procedure counts with both machines, but in the normal range. Similarly, the study by Jaipain et al. showed that post-procedure data for the Amicus™ were lower than the Trima Accel®12 This may be caused by the use of ACD-A in both machines afforded equal acid citrate dextrose (ACD-A) concentration (10:1), and their price was included in the kit price. The machines yielded equivalent anticoagulant ratios, but our data showed that the Amicus™ used a greater volume than the Trima Accel®, because of the high volume of whole blood processed, which would also enhance this factor. In a previous study by Picker and colleague5, it was found that the single-stage channel of the Trima Accel® allowed platelets to be sandwiched between plasma and red blood cells, thus avoiding continued contact with the surface of the plastic bag, allowing for a reduced anticoagulant ratio. The study by Burgstaler and associates11 showed the Trima Accel® used significantly less anticoagulant than the Amicus™. However, the Trima Accel® was also faster than the Amicus™ because the production time of the Amicus™ instrument, which isolates platelet concentrates by sedimentation against the collection bowl, included the process of transferring the product from the centrifuge to the storage bag. This transferred required additional time. In our study, the Trima Accel® was the fastest, but the Amicus™ was the most efficient (80%). Burgstaler and associates also reported greater efficiency with the Amicus™ (86%), while the Trima Accel® was faster11. However, Picker and coworkers found equivalent collection efficiency for the Amicus™ (74%) and Trima Accel® (74%), but the Trima Accel® was faster5. In the present study, the efficiency value for the Trima Accel® (76%) was similar to previous reports (71-76%)4- 11 13. The collection-efficiency factor depends upon pre-procedure donor characteristics; total platelet volumes processed and platelet yields also affected efficiency values. Our data showed the Trima Accel® produced the lowest volume of residual white blood cells per unit. Differences in low-range residual white blood cells may affect patient outcomes, according to currently established indications for white blood cell reduction; human leukocyte antigen-induced alloimmunization, febrile non-hemolytic transfusion reactions, and risk of transmitting cytomegalovirus infection14. The apparatus flagged the product with the highest white blood cell count with an alarm due to red blood cell spillover. It was necessary to count residual white blood cells for confirmation. In the present study, the Amicus™ platelet volumes and platelet yields were significantly higher than the Trima Accel®. Both Trima Accel® and Amicus™ predicted platelet yields of 9.5, but the actual platelet yields were > 9.5 (18 (100.0%)). Moreover, the triple-dose platelet product after division into its three component units met the AABB requirements, with at least 90% of units sampled containing >3.0 x 1011 platelets6. We performed a paired study with the same donors, to compare the Trima Accel® with the Amicus™, and it showed that donor safety for the two apheresis machines was good, with no severe adverse effects. Confirming previous reports, Moog, R.1 concluded that the implementation of a triple-dose platelet-collection program is rarely associated with post-plateletpheresis counts below critical values (P < 2%), or apheresis-related adverse reactions-mild citrate toxicity, circulatory reactions, and discontinuation due to venous flow problems may increase in triple-dose platelet apheresis. A previous study by Heuft HG and associates4, found that adverse donor reactions to double-doses and triple-dose platelet collections involved no serious adverse effects, but adverse donor reactions were observed in 265 of 2,249 (11.8%) plateletpheresis procedures for all donor reactions was mild adverse effects. However, moderate reactions resulting in discontinuation for several reasons were rarer and were clearly more frequent in triple-dose platelet collections (6.4%) than double-dose (2.3%), with venous complications or donor compliance problems and citrate toxicity being the major reasons for discontinuation. Hence, the fact that a significant number of donors might not initially have been used to the new procedure might be an important factor. The other factor is that increased numbers of triple-dose donations increased the risk of adverse donor reactions. To assess donor satisfaction, donors were interviewed during the apheresis procedure and were asked to fill out a questionnaire immediately after donations had been completed. Donors who expressed a preference for donating again with the Trima Accel® rather than the Amicus preferred a shorter processing time. Donors who choose the Amicus™ favored the smooth draw and return flow.
This study had some weaknesses: all donors were male (100%), and thus did not represent the diversity of regular donors, who are both male and female. The adverse effects and satisfaction
Comparison of triple-dose single-donor platelet collection by the Amicus™ and the Trima Accel blood-cell separators. questionnaire should be compared with caution, especially because donors could not be masked with respect to the machines and the number of donors evaluated was low. However, sample-size calculation was based on the nQuery program, so the data were deemed adequate for statistical calculations.
In conclusion, triple-dose plateletpheresis collection is the most economical choice for Thailand, because each donation uses a disposable kit for triple-unit plateletpheresis collection. In addition, laboratory costs were reduced threefold. Thus, the patient had more opportunity to access plateletpheresis at low cost, low risk of viral disease transmission, and lower risk with allogenic transfusion. Our data demonstrated that triple-dose plateletpheresis was safe with the selected donors. Both cell separators were efficient collectors of triple-dose platelets containing >3.0 x 1011 platelets. The platelet yields of the products met AABB guidelines. Further studies confirming the clinical effectiveness of triple units should be conducted.
ACKNOWEDGEMENTS
The success of this study is due to the attentive support of Dr. Viroje Chongkolwatana, my major advisor, and the National Blood Centre, Thai Red Cross Society, who provided support and encouragement throughout the study.
REFERENCES
1. Moog R. Feasibility and safety of triple dose platelet collection by apheresis. J Clin Apher 2009;24:238-40.
2. National Blood Center, Thai Red Cross Society. Annual report 2013. Bangkok:Chulalongkorn University Printing House;2015.
3. Popovsky MA. Multicomponent apheresis blood collection in the United States. Transfus Apher Sci 2005;32:299-304.
4. Heuft HG, Moog R, Fischer EG, Zingsem J, the German and Austrian plateletpheresis study group. Donor safety in triple plateletpheresis: results from the German and Austrian Platelet-pheresis Study Group multicenter trial. Transfusion 2013;53:211-20.
5. Picker SM, Radojska SM, Gathof BS. Prospective comparison of high-dose plateletpheresis with the latest apheresis systems on the same donors. Transfusion 2006;46:1601-8.
6. Roback JD, Combs MR, Grossman BJ, Hillyer CD,eds. Technical manual. 16th ed. Bethesda: MD: American Association of Blood Bank, 2008.
7. Moog R, Zeiler T, Heuft HG, Stephan B, Fischer EG, Kretschmer V, et al. Collection of WBC-reduced single-donor PLT concentrates with a new blood cell separator: results of a multicenter study. Transfusion 2003;43:1107-14
8. Landau S, Everitt BS, eds. A handbook of statistical analyses using SPSS. Florida: CRC Press LLC, 2004.
9. Wagner WE. Using IBM SPSS statistics for research methods and social science statistics. 5th ed. Channel . SAGE, 2014.
10. Food and Drug Administration. Guidance for industry and FDA review staff: collection of platelets by automated methods.Washington, DC. U.S. Department of Health and Human Services 2007.
11. Burgstaler EA, Winters JL, Pineda AA. Paired comparison of Gambro Trima Accel versus Baxter Amicus single-needle plateletpheresis. Transfusion 2004;44:1612-20.
12. Jaipian J, Chuansumrit A, Chongkolwatana V, Kunakorn M, Kitpoka P. Comparison of efficacies of double dose single donor platelet by blood cell separators. J Hematol Transfus Med
2013;23:121-8.
13. Fontana S, Mordasini L, Keller P, Taleghani BM. Prospective paired crossover comparison of multiple single-needle plateletpheresis procedures with the Amicus and Trima Accel cell separators. Transfusion. 2006;46:2004-10.
14. Zingsem J, Zimmermann R, Weisbach Volker, Glaser A, Bunkens H, Eckstein R. Comparison of a new WBC-reduction system and the standard plateletpheresis protocol in the same donors. Tranfusion 2001;41:396-400.


