Molecular diagnosis of synovial sarcoma:A comparative study with histologic correlation in Ramathibodi Hospital
Artit Jinawath1, M.D., Ph.D., Witoo Klabtawee1, M.D.,
Kaettipong Kamprerasart1, M.Sc., Lalida Arsa1, M.Sc.,
Vorachai Sirikulchayanonta2, M.D.
1Department of Pathology, Faculty of Medicine Ramathibodi Hospital,
Mahidol University, Bangkok 10400, Thailand.
2Faculty of Science, Rangsit University, Pathumthani 12000, Thailand
Correspondence: Artit Jinawath Artit Jinawath M.D., Ph.D.
Department of Pathology, Faculty of Medicine, Ramathibodi Hospital,
Mahidol University, Bangkok, 10400, Thailand. Phone:662-201-1667;
E-mail: artitjp@yahoo.com
Received: 10 January 2016; Accepted 2 February 2016
ABSTRACT
Objective: Synovial sarcoma (SS) is a relatively common sarcoma of soft tissue arising in extremities of young adult, which is often diagnosed by histology and immunohistochemistry (IHC). Synovial sarcoma can be found in various locations and may cause diagnostic dilemma in tumors arising in unusual locations. In this study, we evaluated the application of molecular test namely SS18/SSX fusion transcripts as an additional diagnostic tool for synovial sarcoma.
Method: We performed the reverse transcription polymerase chain reaction (RT-PCR) to detect the presence of SS18/SSX fusion transcripts in 22 tumors from various anatomical sites previously diagnosed as SS or having SS in the differential diagnoses from the years 2010 to 2014.
Result: Of 22 cases analyzed, 14 cases (63.6%) were positive either for SS18/SSX1 or SS18/SXX2 fusion transcripts by RT-PCR. The remaining 8 cases (36.4%) were negative for both SS18/SSX1 and SS18/SSX2 fusion transcripts. Among 14 cases with positive molecular testing results, 12 cases were previously diagnosed as SS (85.7%), whereas the other 2 cases were originally diagnosed as other types of sarcoma (14.3%). On the other hand, from 17 cases which were initially diagnosed as SS by histology and IHC, 12 cases (70.5%) were positive for molecular test using SS18/SSX fusion transcripts, whereas 5 cases were negative.
Conclusion: We found that the detection of SS18/SSX fusion transcripts by RT-PCR is a valuable method to confirm the diagnosis of SS, especially in those difficult cases arising in uncommon sites or presenting with unusual histology and unconventional immunohistochemical profiles.
Key words: Synovial sarcoma, Reverse transcription polymerase chain reaction, Immunohistochemistry, fusion transcript
Running title: Molecular diagnosis of synovial sarcoma
Abbreviations: SS = synovial sarcoma, RT-PCR = reverse transcription polymerase chain reaction, IHC = immunohistochemistry
INTRODUCTION
Synovial sarcoma (SS) is an aggressive sarcoma which comprises approximately 5-10% of soft tissue sarcoma and frequently arises in deep soft tissue of extremities, particularly around the large joints of adolescents and young adults1-3. However, all age groups4-5 and wide variety of anatomical locations including lung, ovary, kidney, mediastinum, breast, head and neck, and pleura can also be affected1-4. Approximately more than 90% of all SS have been shown to contain a reciprocal translocation between chromosome X and 18; t(X;18)(p11.2;q11.2) leading to the formation of SS18/SSX fusion genes4, 5. Up to 98% of SS cases were shown to harbor either SS18/SSX1 (type 1) or SS18/SSX2 (type 2) fusion transcript by specific molecular techniques4, 5 6. Rare cases of SS were found to have another type of specific gene fusion; SS18/SSX4 fusion gene7. In addition, there was a recent study that revealed a novel chromosomal rearrangement of chromosome X and 20; t(X;20) in one case of SS8.
There are two major morphological subtypes of SS: the more common monophasic subtype composed of short fascicles of uniform spindle cells, and biphasic type comprising epithelial and spindle cell components in varying proportion2, 9. Other two less common variants are monophasic epithelial and poorly differentiated SS2,9.
The diagnosis of SS is often based on microscopic and immunohistochemical evaluation. Typically, about 90% of all SS cases focally express cytokeratin in the spindle cell component. Epithelial membrane antigen is found to express more often and widely than cytokeratin2, 10. S100 and CD99 may be detected in 30% and 62% of cases, respectively, while CD34 is usually negative2,10. Bcl2 protein is diffusely expressed in all SS, especially in spindle cells11. Diagnosis of biphasic SS usually poses less difficulty, although this variant shares morphologic features with mesothelioma, mixed mullerian tumors and adenocarcinoma. However, monophasic SS can be difficult to be distinguished from other spindle cell tumors based on histologic and immunohistochemical profiles. The differentiation of monophasic SS from other tumors, such as malignant peripheral nerve sheath tumor (MP-NST), fibrosarcoma, undifferentiated/unclassified sarcomas, solitary fibrous tumor (SFT) and mesothelioma can be challenging, especially when the tumors arise in uncommon sites4, 5 12. Therefore, molecular analysis for the detection of SS18/ SSX fusion transcripts has been shown to be a very useful tool for making the diagnosis of SS in difficult cases4, 5 6 11.
In this study we investigated the presence of SS18/SSX fusion type 1 and 2 by reverse transcription polymerase chain reaction (RT-PCR) technique in 22 formalin-fixed paraffin-embedded tumor tissue (FFPE) from the archive of the Department of Pathology, Ramathibodi hospital, which were previously diagnosed as SS or having SS in one of the differential diagnoses by histology and IHC. This study was also aimed to develop and apply the molecular technique as a diagnostic tool for routine service, and to reclassify the diagnosis of the cases which were negative for SS18/SSX fusion transcripts.
MATERIALS AND METHODS
The study was approved by Committee on Human Rights Related to Research Involving Human Subject, Faculty of Medicine Ramathibodi Hospital, Mahidol University on 9th April 2008.
Twenty two cases diagnosed as SS (cases No.1-17) or having SS in one of the differential diagnoses (cases No.18-22) from the years 2010 to 2014 were retrieved from the files of the Department of Pathology, Ramathibodi hospital. The clinicopathological data of these cases were summarized in Table 1. The histological features varied from biphasic tumor, monomorphic spindle cells tumor, round cells tumor, myxoid and pleomorphic tumor. Diagnosis was made by histologic examination with additional IHC. IHC staining was performed by Bond-max TM immunostainer (Leica Microsystems, UK) using Bond detection system. The tumor cases were stained with these antibodies; Bcl2, CD34, CD99, CK AE1/AE3, EMA, and S100, except for the case No.8 which IHC were performed only for CK AE1/ AE3, Desmin, Muscle actin and Smooth muscle actin. Additional IHC staining with other antibodies were performed according to the differential diagnoses.
Real time - Reverse transcription polymerase chain reaction (RT-PCR)
The RNA was extracted from FFPE tissues with High Pure FFPET RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany). Reverse Transcription of total RNA was performed using 2-4 jng of total RNA, random hexamers and SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, California) to generate complementary DNA. RT-PCR reactions were performed in a total volume of 20 pi. containing 2 pi of cDNA. IX iTaq universal probe supermix (BIO-RAD, CA, US), 0.3 pM of forward primer, 0.3 pM of reverse primer, and 0.05 pM of SS18/ SSX1 probe or 0.05 pM of SS18/SSX2 probe.
The real time PCR cycle conditions consisted of an initial denaturation step at 950 C for 2 min, followed by 40 cycles of 15 sec at 950 C, 60 sec at 600 C and detection fluorescence signal ofFAM and HEX channels. The sequences of primers and fluorescence-labeled probes are as follows: Forward primer : 5 ‘ AGA GGC CTT ATG GAT ATG ACC A 3 ’ ,
Reverse primer : 5 ‘ CRT TTT GTG GGC CAG ATG C 3 ’ ,
SS18/SSX1 probe : 5 ‘ [FAM] TCC CTT CGA ATC ATT TTC GTC CTC TGC T [TAM] 3 ’ , and SS18/SSX2 probe : 5 ‘ [HEX] TCT GGC ACT TCC TCC GAA TCA TTT CCT T [TAM] 3 ’ .
The PCR products were confirmed by direct sequencing for the presence of SS18/SSX fusion transcripts.
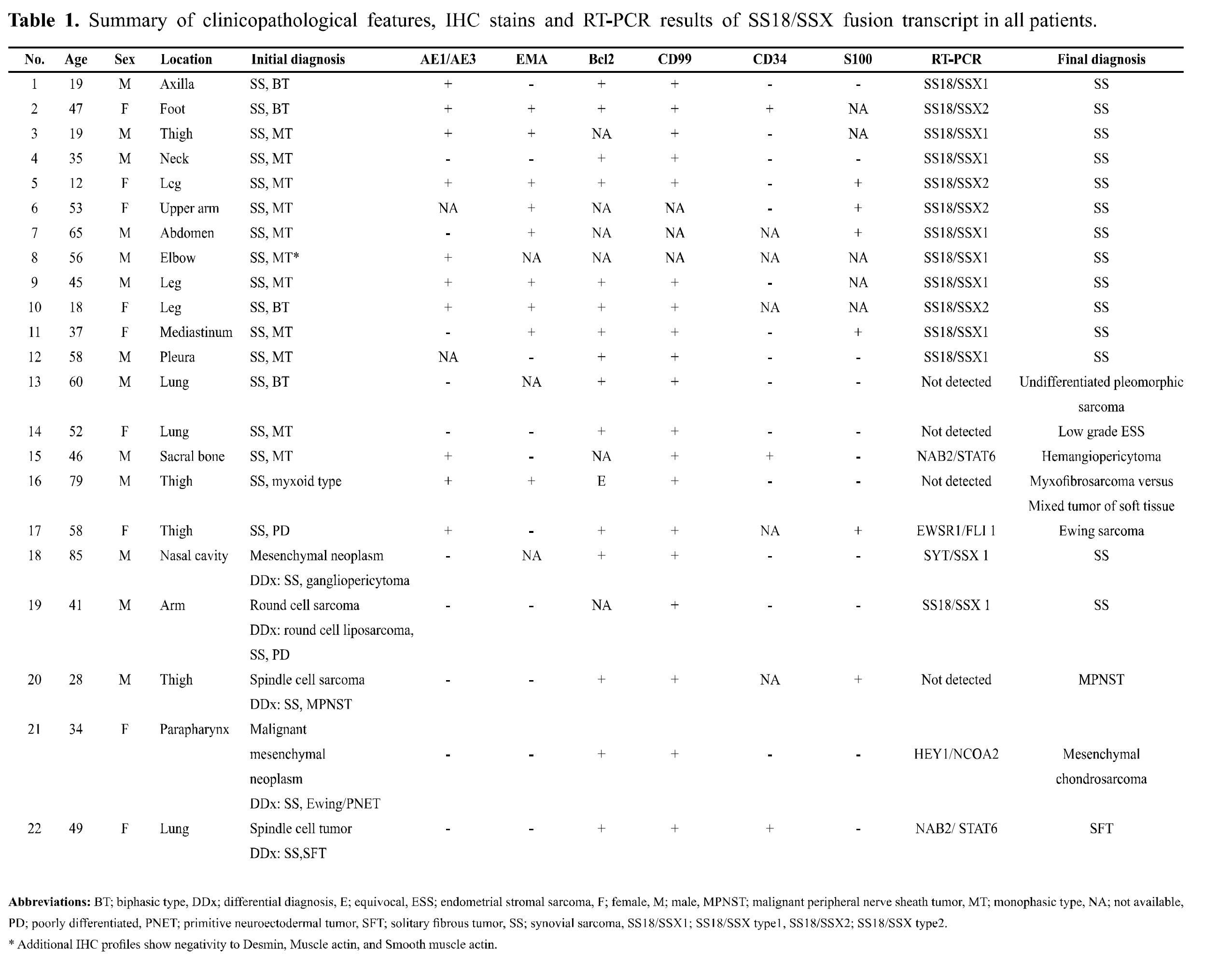
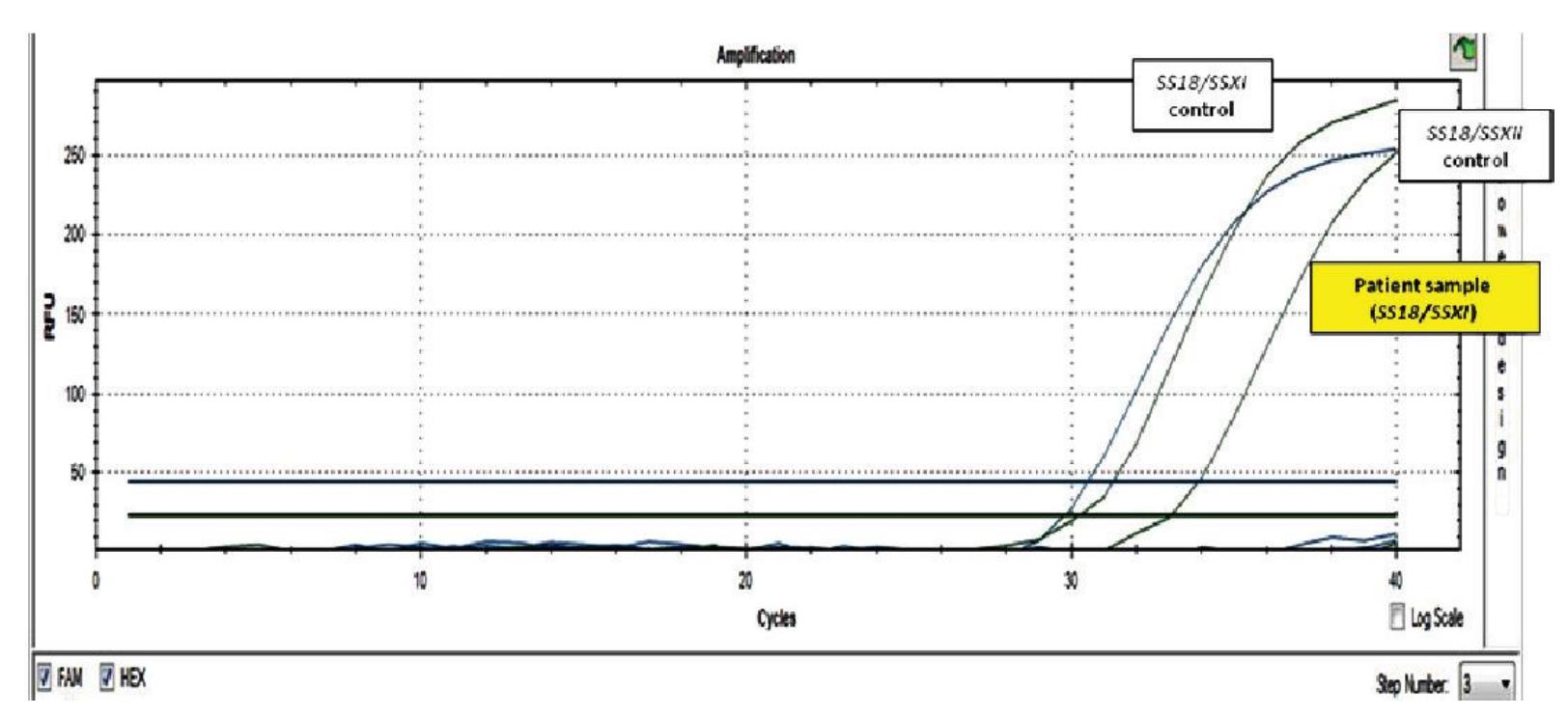
Figure 1 RT-PCR result of SS18/SSX fusion gene. Positive control cDNA of SS18/SSX type 1 and type 2 were included. The presence of SS18/SSX type 1 and 2 transcripts was shown as amplification curve of fluorescent signals of FAM and HEX, respectively. The tested sample was positive for SS18/SSX type 1.
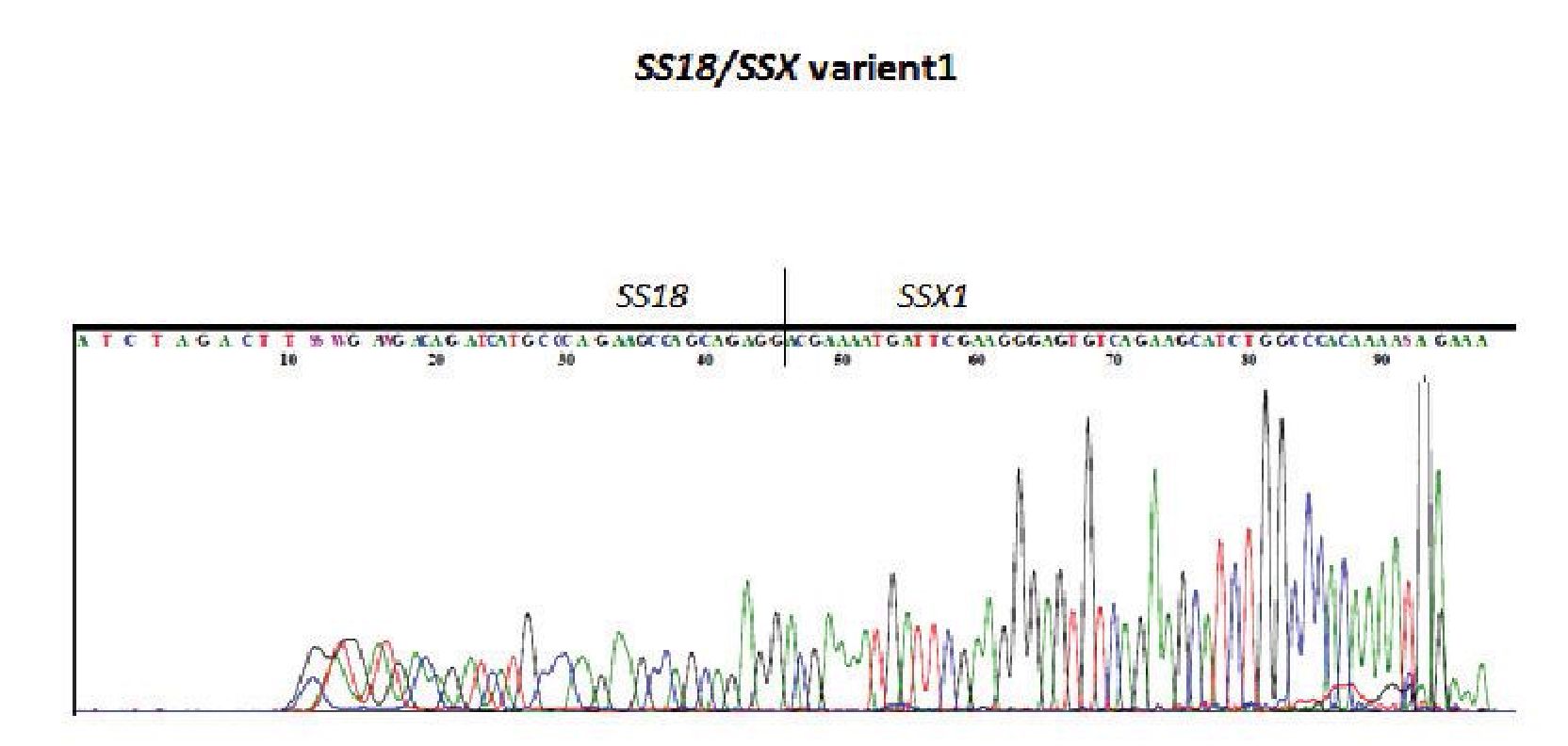
Figure 2 Direct sequencing of the PCR product which was positive for SS18/SSX type 1 by RT-PCR.
RESULT
Clinical and Histologic features
Seventeen cases were diagnosed as SS (cases No. 1-17), and five cases were diagnosed as unspecified mesenchymal neoplasm/spindle cell sarcoma which included SS in one of the differential diagnoses (cases No. 18-22) (Table 1). Almost all cases were typically characterized by expression of CK AE1/AE3 and/or EMA, Bcl2, and CD99. S100 expression was found in few cases. CD34 expression was negative except for 2 cases (No. 2 and No. 15).
Patients presented at the ages between 7 and 85 years with the mean of 45.3 years. There was slightly male predominant (male/female ratio = 1.18:1). The lower extremities were the most common site (8 cases), followed by intrathoracic organs (lung, pleura and mediastinum) (5 cases), upper extremities (3 cases), head and neck (3 cases), trunk (2 cases) and bone (1 case) (Table 1).
In order to confirm the histologic and IHC diagnosis of cases No.1-17, the specimens were tested for fusion transcripts of SS18/SSX1 and SS18/SSX2 by RT-PCR. Of the total 17 cases analyzed, 12 cases (70.6%) were positive for SS18/ SSX1 or SS18/SXX2 fusion transcripts by RT-PCR (Table 1). Figure 1 and 2 demonstrated an example of a case with SS18/SSX1 fusion positive by RT-PCR, which was subsequently confirmed by direct sequencing. The remaining 5 cases (29.4%) were negative for both SS18/SSX1 and SS18/SSX2 fusion transcripts. Interestingly, in our study, all cases that originated from extremities and trunk with classical histological morphology and typical IHC profiles (cases No. 1-10) were 100% positive for SS18/SSX1 or SS18/SSX2 fusion transcripts.
For the 5 cases which were initially diagnosed as SS by histology and IHC but the subsequent SS molecular testing was negative (cases No. 13-17), the histology and IHC results were reviewed and reclassified as follows:
No. 13: the case was a 60-year-old male with a mediastinal mass which showed pleomorphic spindle cell tumor with glandular structures, resembling biphasic synovial sarcoma (Figure 3.1). IHC showed negative for CD34 and S100 but positive for CK AE1/AE3, Bcl2 and CD99. However, TTF1 was positive for the glandular epitheliums of the entrapped bronchi which were mis-interpreted as glandular formation in biphasic SS. The case was also negative for both types of SS18/SSX fusion transcripts. In this case, the patient had previous history of retroperitoneal sarcoma. The case was finally diagnosed as metastatic undifferentiated pleomorphic sarcoma.
No. 14: the case was a 52-year-old female with a lung mass which comprised monophasic uniform densely-packed short spindle cells arranging in fascicular pattern (Figure 3.2). The IHC study showed positive staining for Bcl2 and CD99, but negative for CK AE1/AE3, EMA, CD34 and S100. Despite negative result to SS18/SSX fusion transcripts by RT-PCR, the case was concluded to be SS during that time. However, 5 years later, the following specimen of uterus and both adnexa were obtained for pathological examination and revealed low grade endometrial stromal sarcoma, morphologically similar to the lesion found in the lung. Therefore, the final diagnosis of this case should be metastatic low grade endometrial stromal sarcoma.
No. 15: the case was a 46-year-old man with a sacral bone mass. This lesion was characterized by patternless spindle cell tumor with staghorn vessels and focal positive IHC for CK AE1/AE3, CD99, and CD34 (Figure 3.3). The case was negative for SS18/SSX fusion transcripts. This patient had previously been diagnosed as atypical meningioma since year 2003. The histologic review of the meningeal specimen suggested hemangiopericytoma. The specimen from sacrum was found to be positive for the NAB2/STAT6 fusion transcripts by RT-PCR the finding of which was compatible with solitary fibrous tumor/heman-giopericytoma. This case was finally diagnosed as metastatic meningeal hemangiopericytoma.
No. 16: the case was a 79-year-old man with a thigh mass. The lesion contained epithelioid and spindle-shaped tumor cells arranging in reticular or trabecular growth pattern in abundant myxoid stroma containing scattered inflammatory cells (Figure 3.4). The IHC staining showed strongly diffused positivity for CK (AE1/AE3), EMA, and CD99, while CD34 and S100 were negative. The case was negative for SS18/SSX fusion transcripts. The diagnosis of this case is still unclear, but most likely to be either low grade myxofibrosarcoma or mixed tumor of soft tissue.
No. 17: the case was a 58-year-old female with a large thigh mass. The histology of the tumor revealed mixed round cells and short spindle cells (Figure 3.5). The IHC profiles showed positivity for CK (AE1/AE3), Bcl2, and CD99. Therefore, this case was diagnosed as poorly differentiated synovial sarcoma. However, the RT-PCR study was negative for SS18/SSX fusion transcripts but positive for EWSR1-FLI1 fusion transcript, which was consistent with Ewing sarcoma. Although this patient was not young at presentation (58 years) but this diagnosis is still possible since Ewing sarcoma may occasionally occur in elderly. Finally, this case was subsequently diagnosed as Ewing sarcoma.
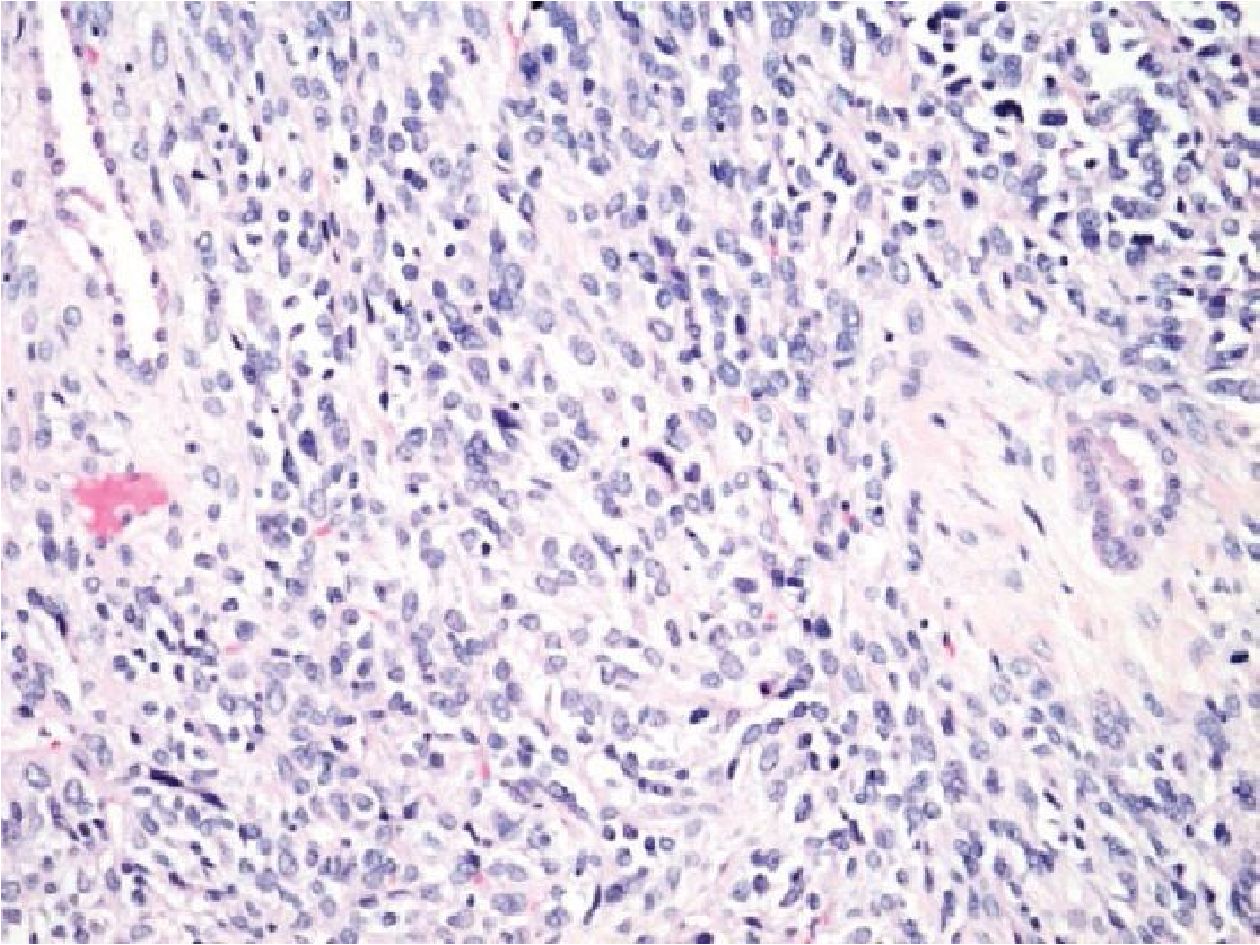
Figure 3.1 Mediastinal mass of a 60-year-old male (H&E stained section original magnification x 200) of the case No. 13, showing pleomorphic spindle cell tumor with glandular structures, resembling biphasic SS. However, the case showed no amplification of SS18/SSX fusion transcripts. This case was finally diagnosed with undiffertentiated pleomorphic sarcoma.
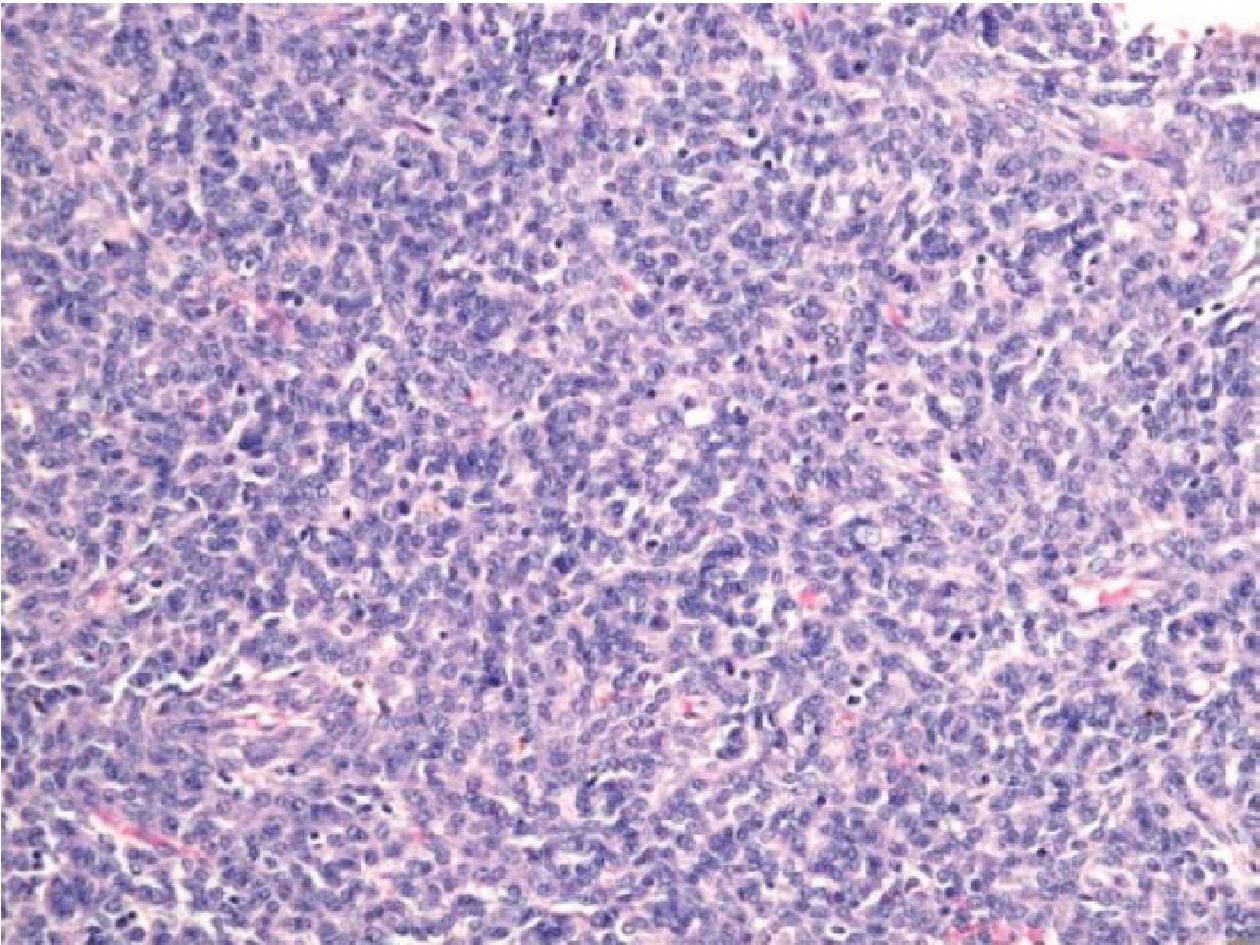
Figure 3.2 Lung mass of a 52-year-old female (H&E stained section original magnification x 200) of the case No.14, showing uniform densely packed spindle cells, resembling monophasic SS, but showed no amplification of SS18/SSX fusion transcripts. This case was proved to be metastatic low grade endometrial stromal sarcoma 5 years later.
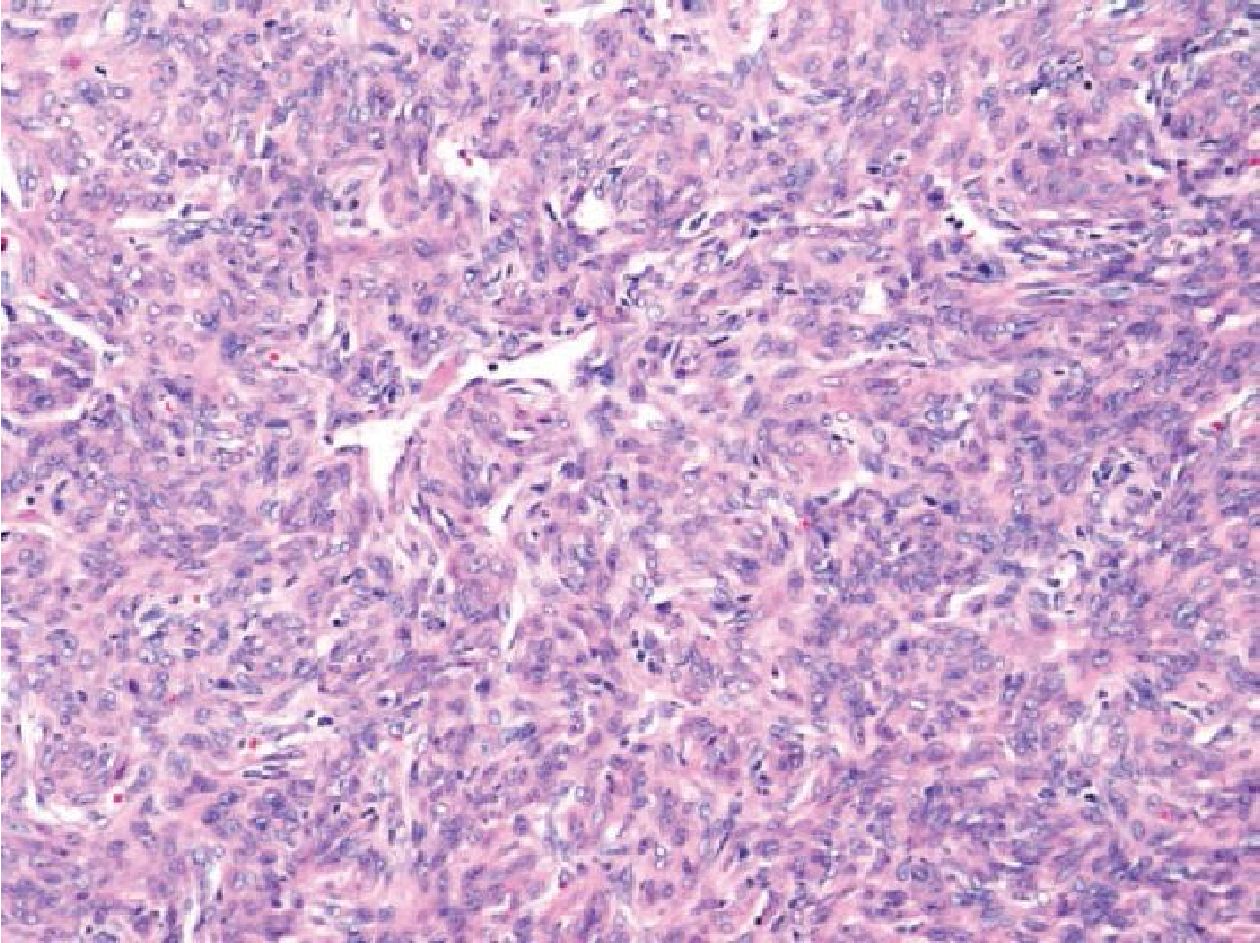
Figure 3.3 Sacral bone mass of a 46-year-old man (H&E stained section original magnification x 200) of the case No.15, revealing patternless spindle cell tumor with staghorn vessels showing negative molecular result for SS18/SSX fusion transcripts but positive for NAB2/STAT6 fusion transcripts. Therefore, this case was finally diagnosed as solitary fibrous tumor/hemangiopericytoma.
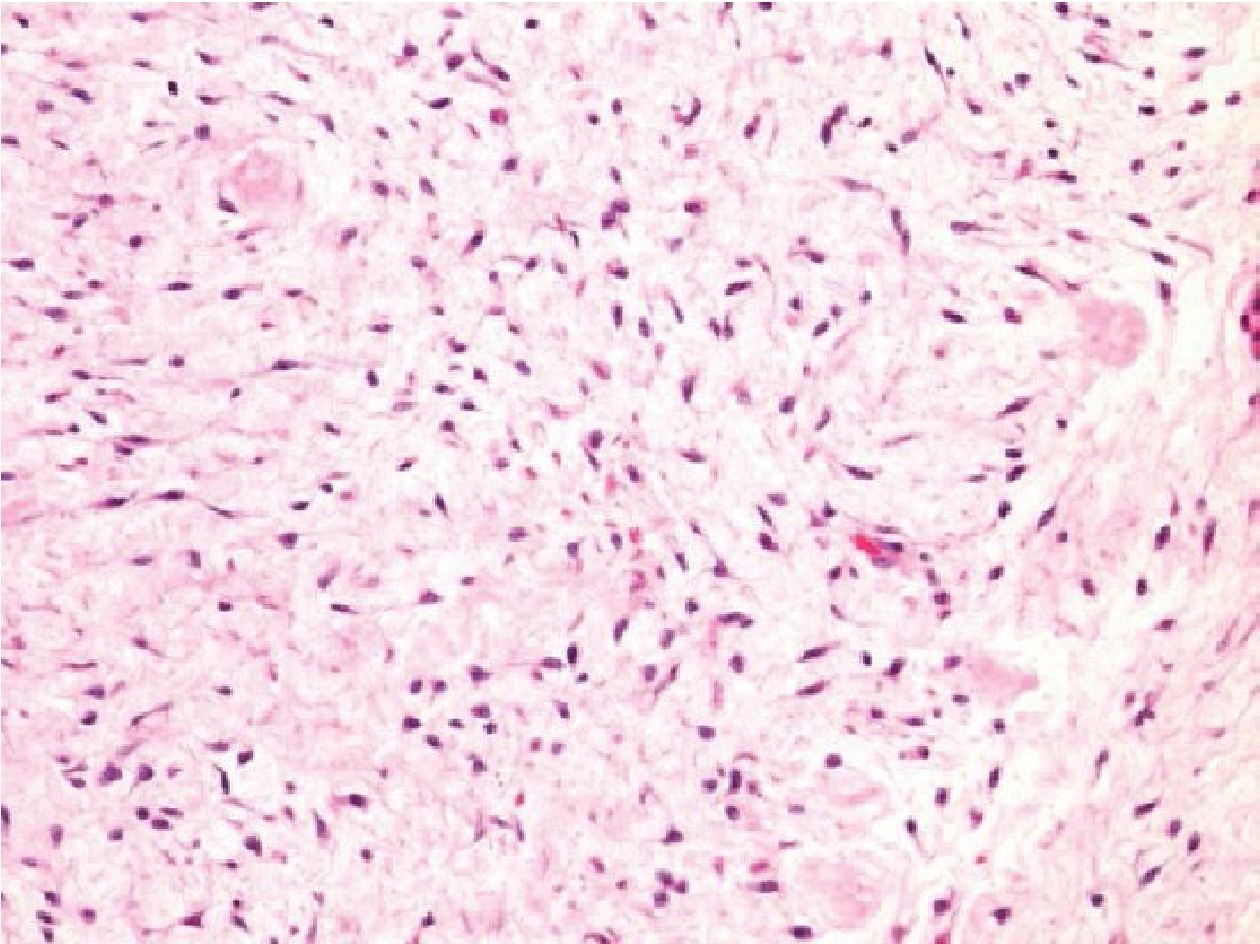
Figure 3.4 Thigh mass of a 79-year-old man (H&E stained section original magnification x 200) of the case No.16, demonstrating epithelioid to spindle shaped cell, arranging in fascicle with abundant myxoid stroma. The case showed no evidence of SS18/SSX fusion transcripts amplification. From histology and IHC profiles, this case was suggestive of low grade myxofibrosarcoma or mixed tumor of soft tissue.
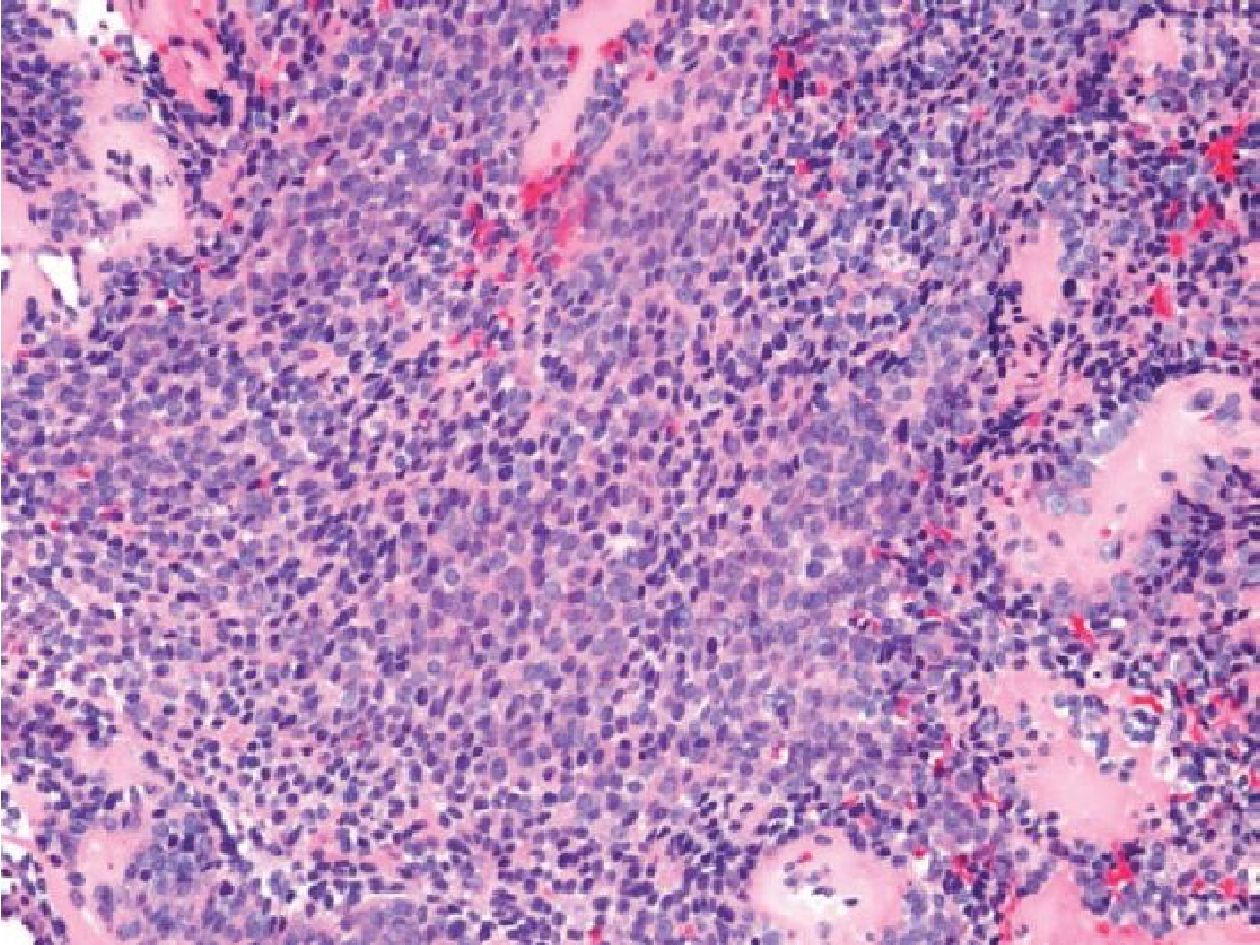
Figure 3.5 Thigh mass of a 58-year-old female (H&E stained section original magnification x 200) of the case No.17. The histology revealed mixed round cells and short spindle cells. The case was negative for SS18/SSX fusion transcripts, but positive for ESWR/FLI1 fusion transcripts. The case was subsequently diagnosed as Ewing sarcoma.
Regarding the 5 cases which had SS in the differential diagnosis (cases No. 18-22) (Table 1), all of them showed monophasic spindle cell sarcoma without glandular or cartilage formation. Three cases (cases No. 20-22) arose in unusual locations including lung, parapharynx and nasal cavity. Further molecular test confirmed the diagnosis of SS in the cases of nasal cavity and arm (cases No. 18-19). Finally the diagnoses had been reclassified and were summarized as follows:
No. 18: the case was an 85-year-old female with nasal cavity tumor. The histology revealed monomorphic spindle cells. IHC profile showed positive staining for Bcl2 and CD99 but negative for CK (AE1/AE3), CD34 and S100. The differential diagnoses were SS and gangliopericytoma. The RT-PCR was positive for SS18/SSX fusion transcript. Thus, the final diagnosis in this case was SS.
No. 19: the case was a large arm mass in 41-year- old man. The histology showed uniform packed round to oval shape cells having irregular nuclei with coarse chromatin. IHC were positive for CD99 but negative for CK (AE1/AE3), Bcl2, S100 and CD34. The differential diagnoses were Ewing sarcoma, round cell liposarcoma, poorly differentiated SS and undifferentiated round cell sarcoma. The molecular test was done and showed positivity for SS18/SSX fusion transcript. Therefore, this case was diagnosed as poorly differentiated SS.
No. 20: the case was a 28-year-old man who presented with a large thigh mass of which the MRI showed fusiform-shaped mass. The histology revealed spindle cell tumor arranging in fascicular pattern with positivity for Bcl2, S100 and CD99 but negativity for CK (AE1AE3) and EMA. Differential diagnoses were SS and MPNST. The negative result for SS18/SSX fusion transcripts in this case suggested that the case was MPNST.
No. 21: the case was a parapharyngeal mass of a 34-year-old female. The histology showed short spindle and small round cells morphology on a small tissue biopsy. The IHC studies revealed positive staining for Bcl2 and CD99, but negative for CK (AE1/AE3), EMA, S100 and CD34. The differential diagnoses were Ewing sarcoma/ PNET, SS and mesenchymal chondrosarcoma. The molecular test showed negative results for SS18/ SSX fusion transcript. However, the following tissue was sent for examination and revealed focal cartilaginous differentiation. The additional RT-PCR also showed HEY1/NCOA2 fusion transcript. In conclusion, this case was diagnosed as mesenchymal chondrosarcoma.
No. 22: the case was a 49- year-old female with lung mass. The histology of the biopsy showed monomorphic spindle cell lesion with diffuse positive IHC staining for Bcl2, CD99 and TLE1, focally positive for CD34 but negative for CK (AE1/AE3), EMA, and S100. Differential diagnoses included solitary fibrous tumor and SS. Molecular test was proved to be solitary fibrous tumor by the detection of NAB2/STAT6 fusion transcript.
DISCUSSION
In this study, we reviewed histology, IHC and investigated the presence of SS18/SSX fusion transcripts in 22 cases which were previously diagnosed as SS or having SS in one of the differential diagnoses. Of the total 22 cases, 10 cases were positive for SS18/SSX1 fusion transcript (type 1) (9 monophasic SS and 1 biphasic SS), 4 cases for SS18/SSX2 fusion transcript (type 2)(2 monophasic SS and 2 biphasic SS), and 8 cases were negative for both SS18/SSX1 and SS18/SSX2 fusion transcripts. There was a report showing that the certain types of SS18/SSX fusion transcripts were associated with the histologic subtype of SS (monophasic or biphasic patterns)13. However, our data could not find correlation between types of SS18/SSX fusion transcripts and histologic subtypes. It should be noted that we have much smaller sample size than the previous study. More SS specimens with molecular test results are necessary for solving this hypothesis.
In the cases with usual presentation of SS such as a biphasic SS or a monophasic SS arising in the extremities, displaying typical SS histology and IHC profiles (positive for CK (AE1/AE3) and/ or EMA, CD99, Bcl2 and negative for CD34); the histologic diagnosis alone has a very high accuracy rate, and molecular diagnosis may not be routinely required. However, the molecular test for SS was proved to be very useful for diagnosis of SS with unusual histology and SS arising in uncommon locations, where many of histologic mimickers of SS have to be excluded. Our RT-PCR assay was able to help correcting the diagnoses of 5 cases (case No. 13-17) which were initially diagnosed as SS by histology and IHC, of which 4 cases were further proved to be undifferentiated pleomorphic sarcoma, low grade endometrial stromal sarcoma, hemangiopericytoma and Ewing sarcoma. Our molecular test for SS also facilitated the diagnosis of other 5 cases (cases No. 18-22) with inconclusive histologic diagnosis and IHC results by excluding SS from its histologic mimickers such as MPNST, mesenchymal chondrosarcoma and solitary fibrous tumor. These results are similar to the previous study4,13 which conclude that molecular test is very helpful and necessary for diagnosing SS in unusual sites (such as visceral organs and bone), or SS with unusual histology.
According to the literature, 90% of all SS cases focally express cytokeratin in the spindle cell component, whereas EMA may express more often and widely than cytokeratin2, 10. However, we could not detect CK (AE1/AE3) or EMA expression in 2 cases which were confirmed to have SS18/SSX fusion transcripts. Interestingly, those 2 cases were monophasic SS and poorly differentiated SS (round cell morphology) which have been reported to be occasionally negative for CK (AE1/AE3) and EMA9. Although, focal positivity to CK (AE1/AE3) and/or EMA was a hallmark for diagnosis of SS, negative IHC for these markers does not necessary rule out SS.
We also detected CD34 positivity in 1 case from all 14 SS18/SSX fusion transcripts positive cases. It is generally accepted that SS is devoid of CD34 expression. However, there was a study showing that CD34 can be positive up to 6% of SS9.
In summary, molecular test for SS may not be routinely required for the diagnosis of SS cases arising in usual locations or having typical histology and IHC profiles. On the other hand, it is proved to be very helpful for the diagnosis of rare SS cases arising in unusual locations, having unusual histology and unconventional IHC profiles, which can be problematic in distinguishing them from other histologic mimickers such as MPNST, Ewing sarcoma, solitary fibrous tumor, undifferentiated pleomorphic sarcoma etc. Our study also revealed that some SS may not always express CK (AE1/AE3) or EMA and, in rare instance, may also express CD34. Finally, despite the use of histologic examination, IHC study and molecular test, diagnosis of SS should be correlated with clinical and radiologic findings as well.
REFERENCES
1. Trassard M, Le Doussal V, Hacene K, et al. Prognostic factors in localizedprimary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 2001;19:525-535.
2. John RG, Andrew LF, Sharon WW. Enzinger and Weiss’s soft tissue tumors. 6th ed. China: Elsevier saunder; 2014.
3. Bergh P, Meis-Kindblom Jm, Gherlinzoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer 1999;85:2596-2607.
4. Coindre JM, Pelmus M, Hostein I, et al. Should molecular testing be required for diagnosing synovial sarcoma? A prospective study of 204 cases. Cancer Cytopathol 2003;98:2700-7.
5. Amary MF, Berisha F, Bernadi Fdel C, et al. Detection of SS18-SSX fusion transcripts in formaline-fixed paraffin-embedded neoplasms: analysis of conventional RT-PCR, qRT-PCR and dual color FISH as diagnostic tools for synovial sarcoma. Mod Pathol 2007;20:482-496.
6. Hostein I, Menard A, Bui BN, et al. Molecular detection of the synovial sarcoma translocation t(X;18) by real-time polymerase chain reaction in paraffin-embedded material. Diagn Mol Pathol 2002;11:16-21.
7. Fritsch MK, Bridge JA, Schuster AE, et al. Performance characteristics of a reverse transcriptase-polymerase chain reaction assay for the detection of tumor-specific fusion transcripts from archival tissue. Pediatr Dev Pathol 2003;6:43-53.
8. Euthimios D, Demetra R, Anastasios K, Anna T, Kostantina F, Nikolaos P, et al. Novel SYT-SSX fusion transcription variants in synovial sarcoma. Cancer Genet Cytogenetics 2009; 195:54-58.
9. Fisher C. Synovial Sarcoma. Ann Diagn Pathol 1998;2:401-402.
10. Suster S, Fisher C, Moran CA. Expression of bcl-2 oncoprotein in benign and malignant spindle cell tumors of soft tissue, skin, serosal surfaces, and gastrointestinal tract. Am J Surg Vorachai Sirikulchayanonta Pathol 1998;22:863-872.
11. Antonescu CR. The role of genetic testing in soft tissue sarcoma. Histopathology 2006;48:13-21.
12. Nicholson AG, Goldstraw P, Fisher C. Synovial sarcoma of the pleura and its differentiation from other primary pleural tumors: a clinicopathological and immunohistochemical review of three cases. Histopathology 1998; 33:508-513.
13. Daneil T, Ctibor P, Jana S, Pavel D. Molecular diagnosis of synovial sarcoma: RT-PCR detection of SYT-SSX1/2 fusion transcripts in paraffin-embedded tissue. Med Sci Monit 2005;11(3): MT1-7.
14. PelmusM, GuillouL, Hostein I, etal. Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t(X;18) (SYT-SSX)-positive cases. Am J Surg Pathol 2002;26:1434-1440.
15. Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Kanabi N, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumour. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWER1 gene. Gene chromosomes cancer 2010; 49:1114-1124.
16. Tsuneyoshi M, Daimaru Y, Hashimoto H, et al. The existence of rhabdoid cells in specified soft tissue sarcomas. Histopathological, ultrastructural and immunohistochemical evidence. Virchows Arch A Pathol Anat Histopathol 1987;411:509-514.
17. Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH et al. Impact of SYT-SSX Fusion type on the clinical behavior of synovial sarcoma: a multiinstitutional retrospective study of 243 patients. Cancer Res 2002;62:135-140.
18. Takenaka S, Ueda T, Naka N, Araki N,Hashimoto N, Myoui A, et al. Prognostic implication of SYT-SSX fusion type in synovial sarcoma: a multi-institutional retrospective analysis in Japan. Oncol Rep 2008;19:467-476.


