Sclerosing Polycystic Adenosis of the Parotid Gland: Report of A Case with Multifocality
Arpa Pornpetchpracha, M.D.* Satit Chaiprasithikul, M.D.**
Vorachai Sirikulchayanonta, M.D.*
*Department of Pathology, Faculty of medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
** Department of Otolaryngology, Eye Ear Nose Throat Hospital, Bangkok, Thailand
Correspondence: Arpa Pornpetchpracha, M.D.
Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University,
270 Rama VI Road, Ratchatewi, Phayathai, Bangkok 10400, Thailand 10400
Tel+662-201-1432, 662-201-1667 E-mail: arpaphorn@hotmail.com
ABSTRACT
Sclerosing polycystic adenosis (SPA) is a rare tumor-like lesion of the salivary gland that bears a histomor-phological resemblance to fibrocystic disease and sclerosing adenosis of the breast. The lesion is widely considered to be a pseudoneoplastic inflammatory process. However, foci of epithelial dysplasia and carcinoma in situ can occur in the lesion. We report a case of 16-year-old Thai female with multifocality of SPA arising in the right parotid gland. Grossly, the masses were well-circumscribed, firm, and whitish yellow discoloration with scattered small cysts. Microscopic examination revealed partially encapsulated masses composed of multiple irregular lobules containing cystically dilated ducts and foci of ductal and acinar proliferation embedded in sclerotic collagenous stroma. There were multiple foci of intraductal epithelial hyperplasia with focal cytological atypia. Immunohistochemical staining for calponin and smooth muscle actin demonstrated intact myoepithelial layer around each ducts and acini, which was an important clue in diagnosis this lesion.
Keywords: Sclerosing polycystic adenosis, SPA, parotid gland, salivary gland
INTRODUCTION
Sclerosing polycystic adenosis (SPA) is a rare tumor-like lesion of the salivary gland that bears a histomorphological resemblance to fibrocystic disease and sclerosing adenosis of the breast. This entity was first described by Smith et al. in 1996. They published a case series of 9 patients from the Armed Forces Institute of Pathology [1]. The etiology, pathogenesis and true nature of SPA are still controversial. Even though the lesion has been widely considered to be a pseudoneoplastic inflammatory process, the debate that it may represent a neoplastic process was raised, particularly after Skalova et al. had succeeded in demonstrating monoclonality of SPA by using a Human Androgen Receptor (HU-MARA) Iocus as a marker [2]. To the best of our knowledge, there have been about 60 cases of SPA published in the international literature [1-23] after the first report. The large majority of cases occurred in the parotid glands and the remainder arose in the submandibular and minor salivary glands [3, 4]. A peak incidence was in the fifth decade of life [3, 4]. Females were affected more frequently than males [1, 4, 5]. The patients typically presented with slow-growing masses in the salivary glands, which were usually solitary, but possibly multiple or co-exist with other benign salivary tumors [3]. However, synchronous bilateral involvement has not yet been reported.
Here, we report a case of a 16-year-old female with multiple SPA in the parotid gland.
CASE REPORT
A 16-year-old Thai female came to hospital with a problem of recurrent enlarged right parotid gland of 3 years’ duration. Four years prior to this admission, she had undergone superficial parotidectomy because of the painless enlarged right parotid gland. At that time the pathological diagnosis was chronic sialadenitis. One year after surgery, she had a mass about 1 cm in diameter at the same site without any symptoms. The lesion gradually enlarged during the period of 3 years and prompted her to see the doctor. The physical examination revealed three separated masses occurring in the right parotid gland. Excisional biopsy of all masses was performed. The specimens were submitted for histopathological examination, and consisted of three well circumscribed, firm, whitish yellow masses measuring 3.8x3x3 cm, 3.3x2.8x2.5 cm and 2.8x2x1.8 cm. Cut surfaces of the masses showed well circumscribed, firm, whitish yellow tissue with scattered tiny cysts ranging from 0.1-0.2 cm in diameters (Fig.l).
Microscopically, all three masses showed similar in histologic appearance. They were partially encapsulated with thin fibrous tissue and consisted of multiple irregular lobules (Fig.2). These lobules contained areas of acinar and ductal proliferation with scattered cystically dilated ducts embedded in sclerotic collagenous stroma (Fig.2). The epithelial cells lining these dilated ducts were usually flattened to cuboidal bland cells (Fig.3). Occasional ducts were lined by other cell types, including vacuolated, apocrine, and foam cells (Fig.4). Foci of intraductal epithelial hyperplasia forming cribriform structure were often observed with focal and mild cytologic atypia (Fig.5). The acinar component revealed prominent coarse eosinophilic cytoplasmic granules (Fig. 6). Focal lymphocytic infiltration presented in the stroma. The immunohistochemical staining for cal-ponin and smooth muscle actin demonstrated an intact myoepithelial layer around each ducts and acini (Fig.7). The histologic and immunohistochemical findings were fulfilled for the diagnosis of sclerosing polycystic adenosis.
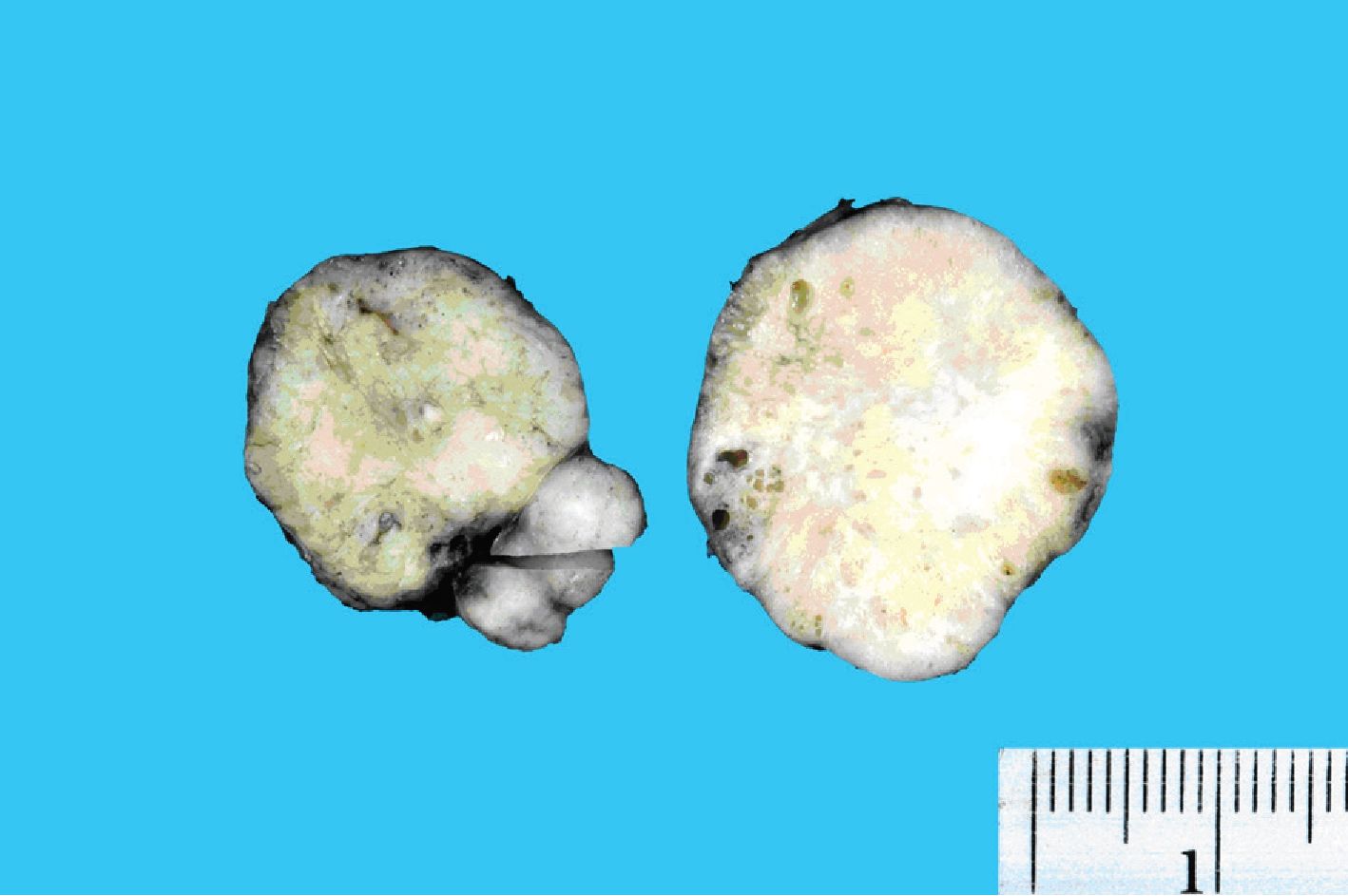
Figure 1. Gross image of the parotid masses demonstrating well circumscribed, firm whitish yellow cut surface with scattered tiny cysts.
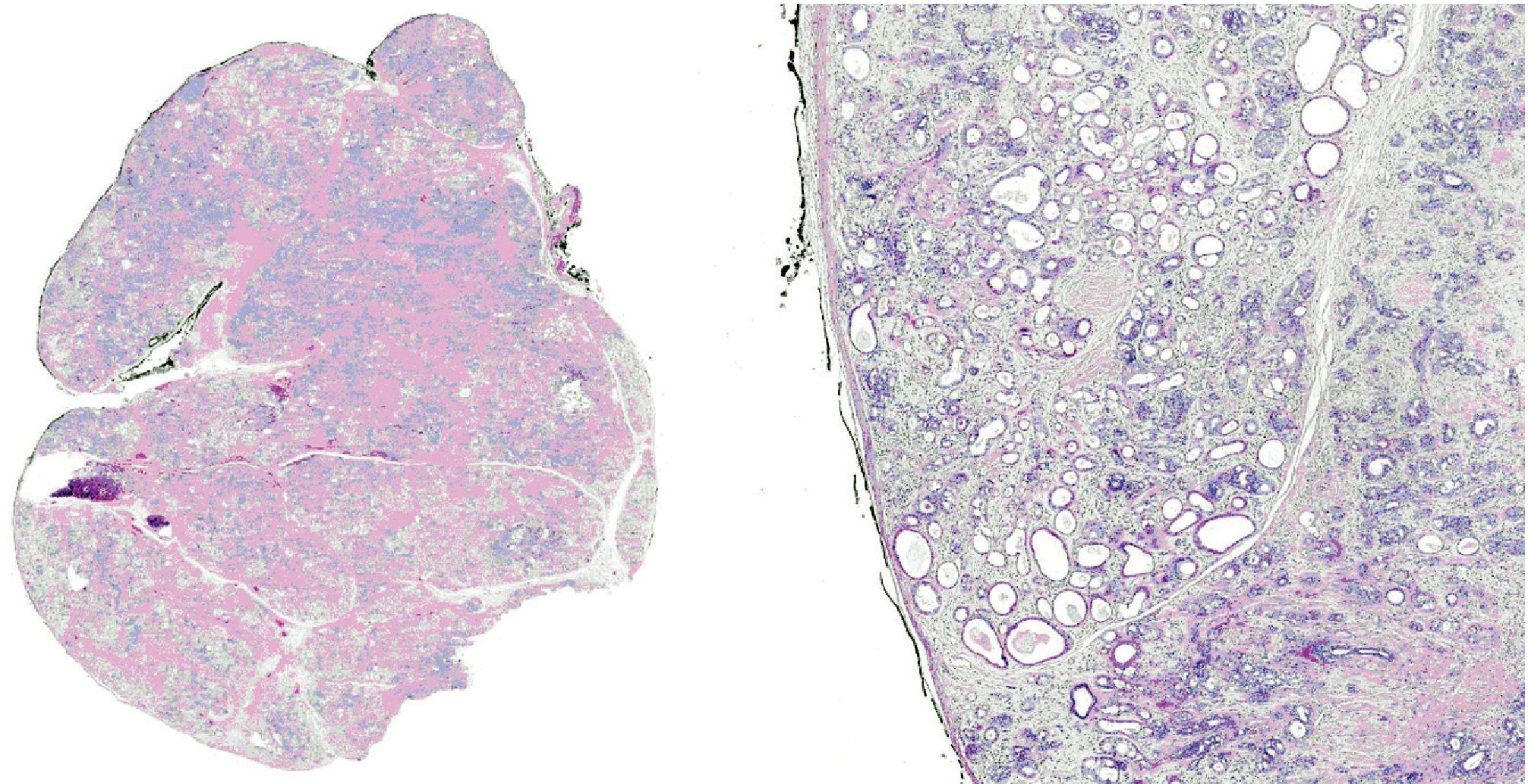
Figure 2. Low-power view demonstrating partially encapsulated mass composed of multiple irregular lobules containing ductal proliferation with cystic dilatation in a background of fibrotic stroma.
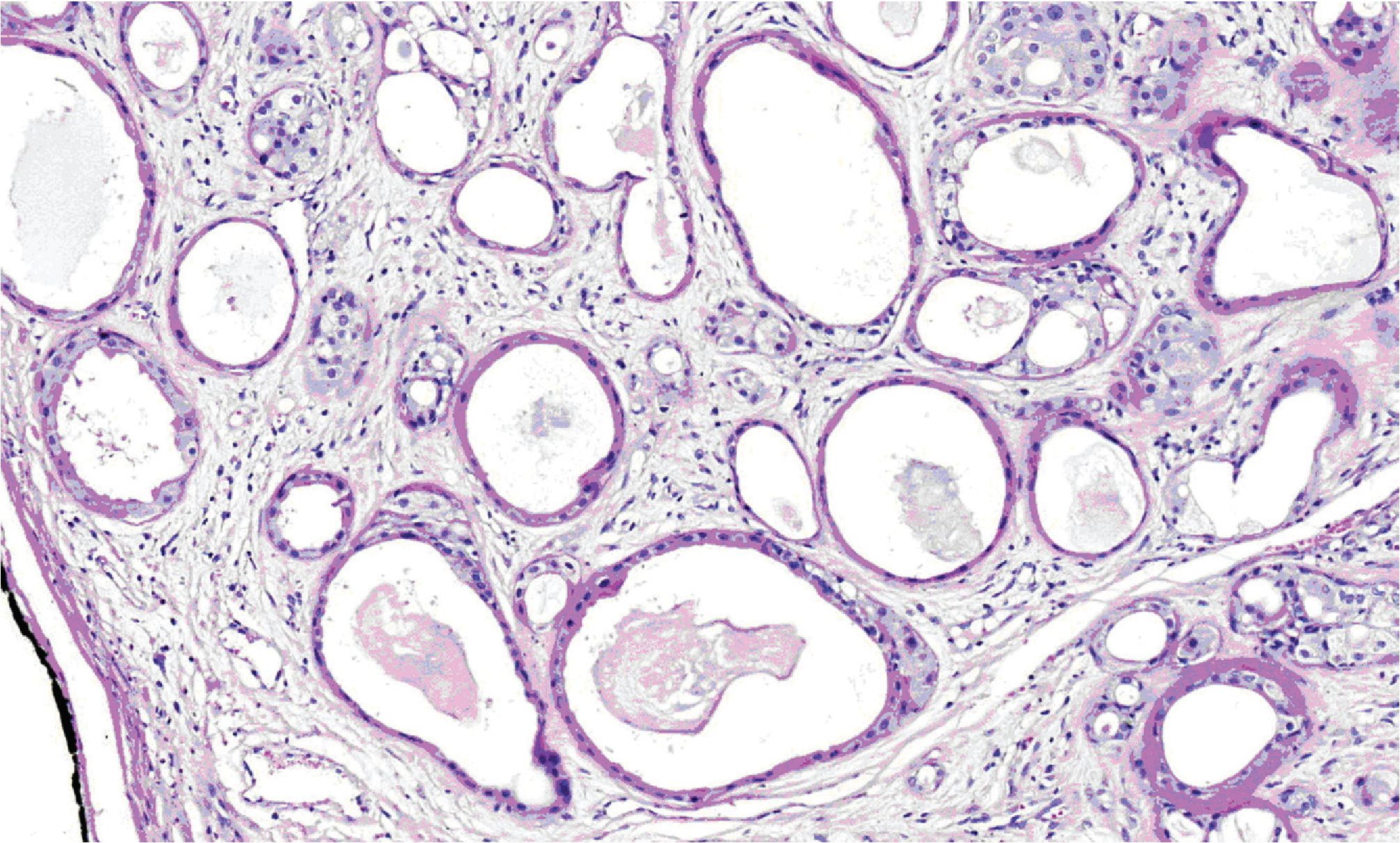
Figure 3. Medium-power view showing cystically dilated ducts lined by flattened to cuboidal epithelium
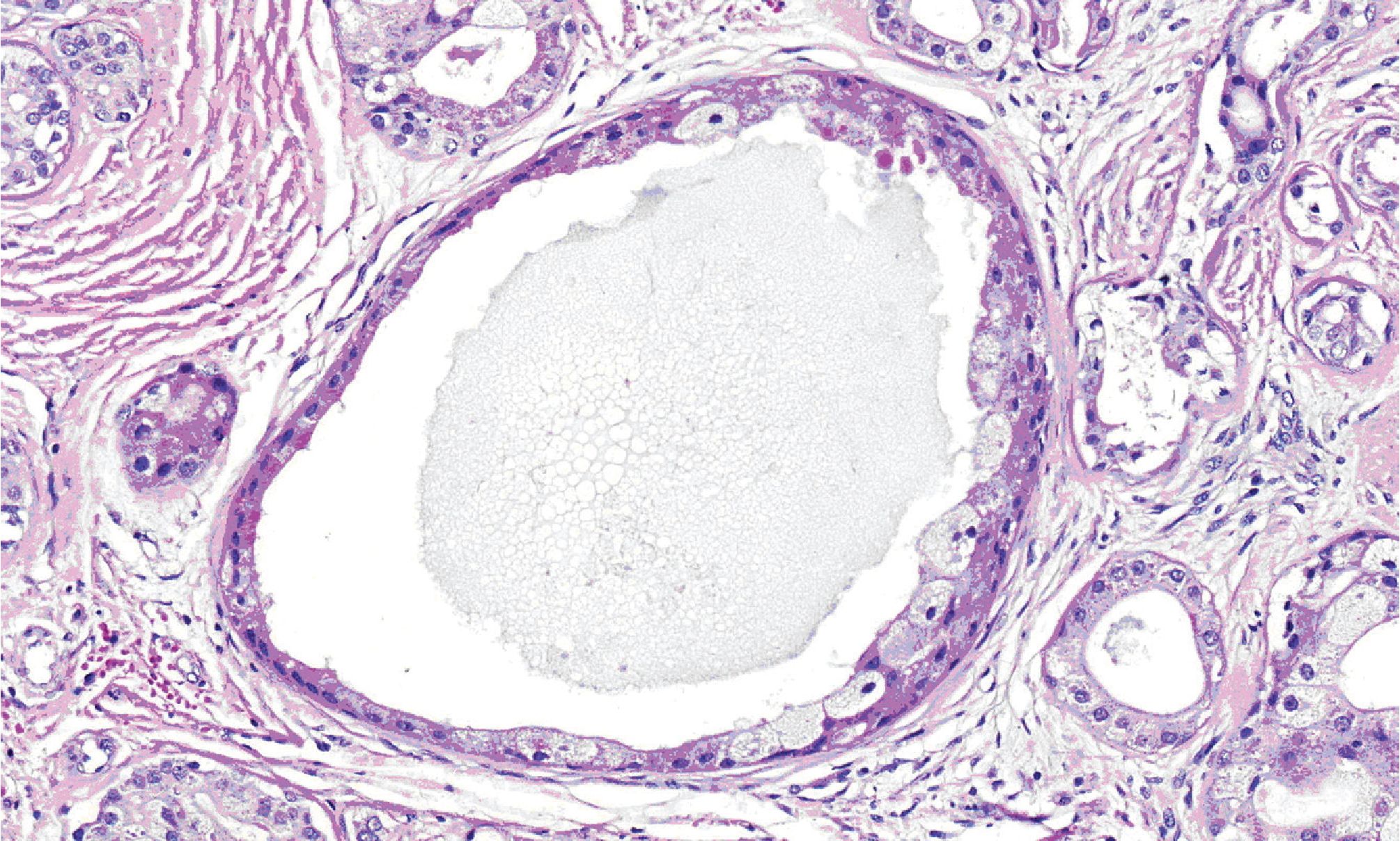
Figure 4. High-power view demonstrating dilated duct with foam cells lining.
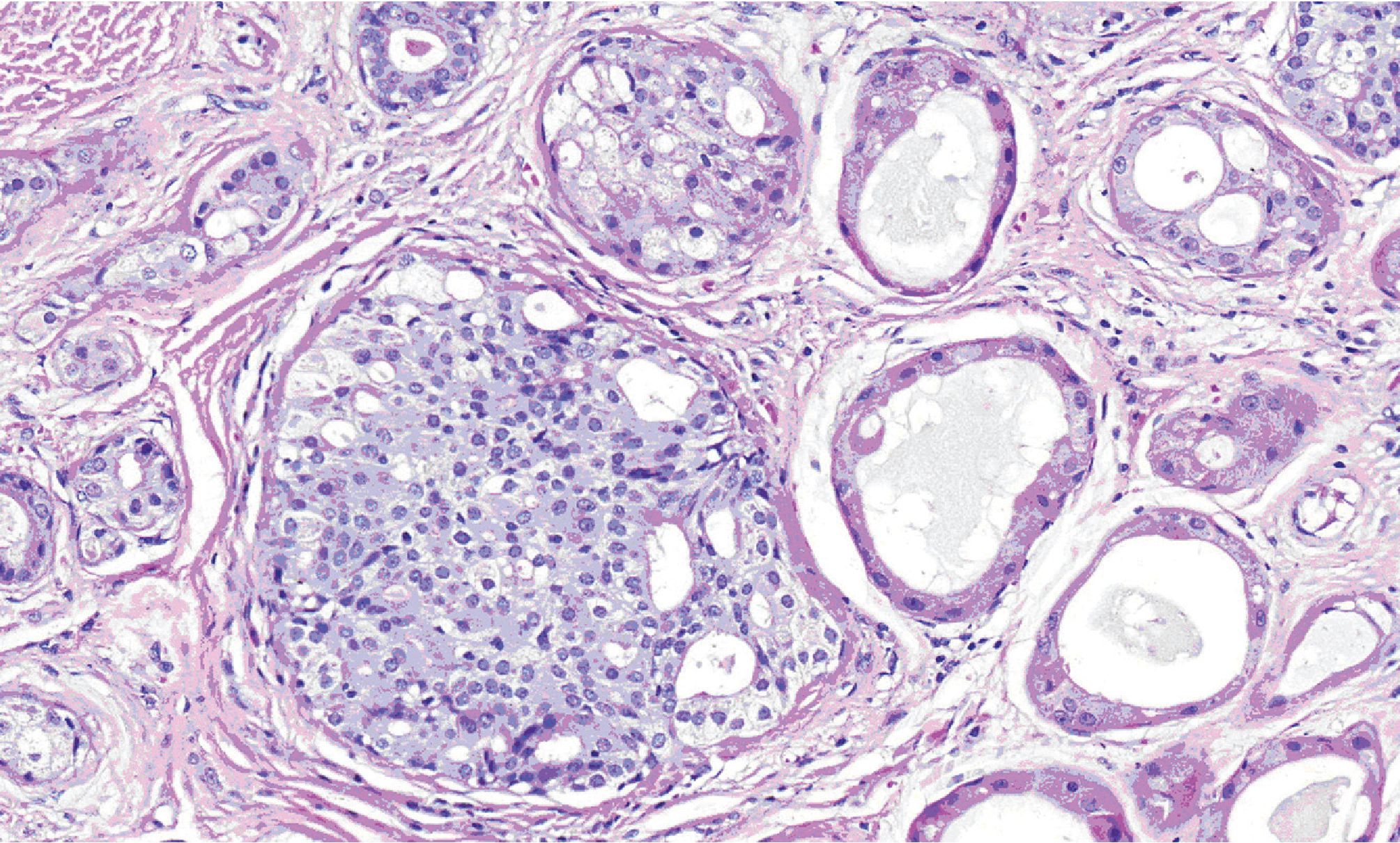
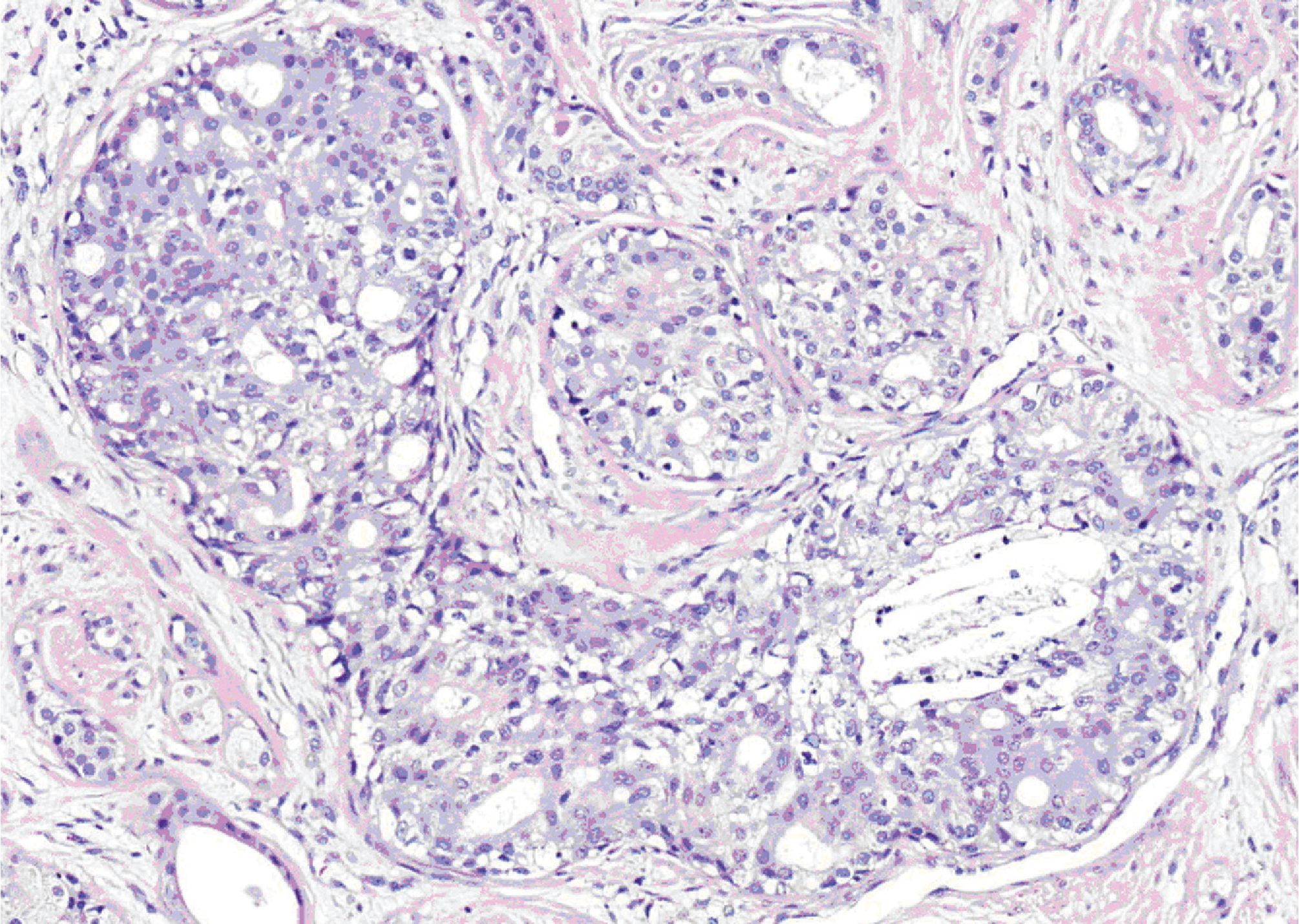
Figure 5. High-power view showing cribriform intraductal epithelial proliferation with mild cytologic atypia.
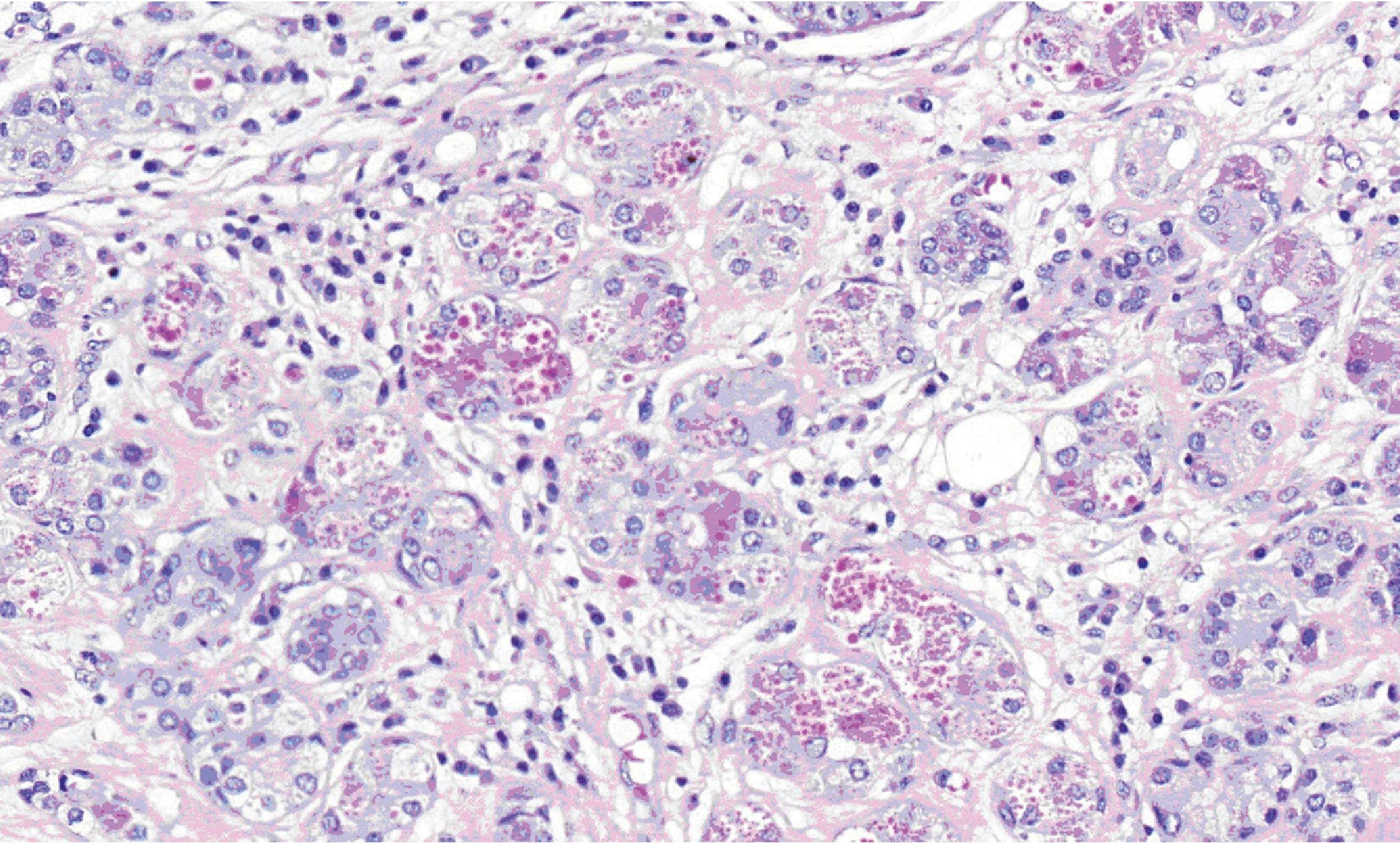
Figure 6. High-power view exhibiting coarse eosinophilic cytoplasmic granules in acinar cells.
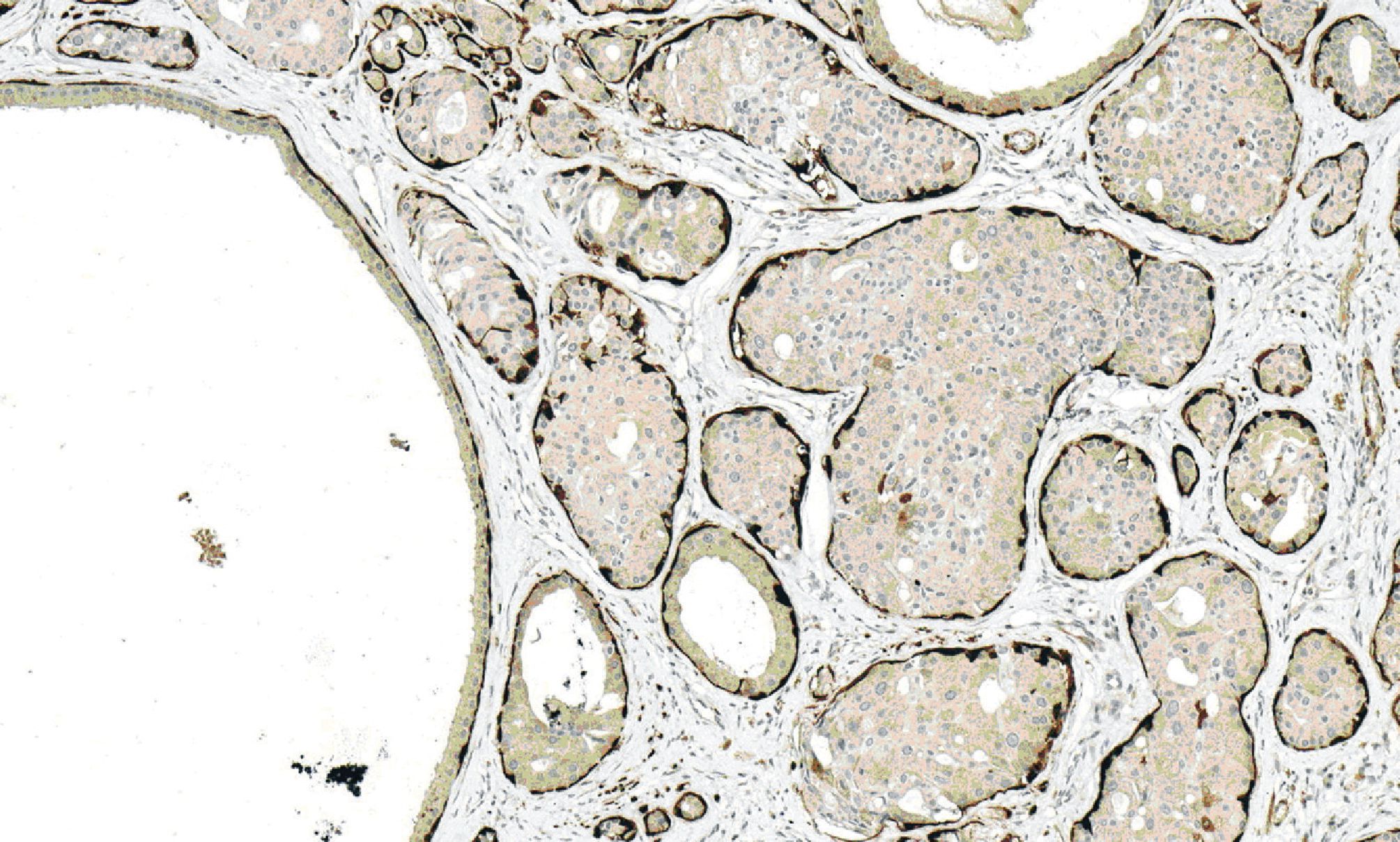
Figure 7. Immunohistochemical staining for calponin demonstrating myoepithelial layers surrounding cysti-cally dilated ducts and hyperplastic ducts.
DISCUSSION
In 1996, Smith et al. first described and named the term “Sclerosing polycystic adenosis (SPA)” for a distinct tumor-like lesion of the major salivary glands. They reported a case series of 9 patients presented with slow-growing masses in the parotid (8 cases) and submandibular glands (1 case) from the Armed Forces Institute of Pathology [1]. In 2006, Gnepp et al. reported the largest series of SPA with a total of 16 cases including the first case of minor salivary gland origin [3]. Up-to-date, there should be 64 cases in the international literature if include our case [1-23]. Based on the clinical features of these reported cases, the patients have a wide range of ages from 9 - 84 years with a peak incidence during the fifth decade of life [3, 6]. The mean age of diagnosis by our review is 45 years. Female is preponderance (37cases of female and 27 cases of male). Size of the lesions ranges from 0.3 - 11.8 cm [3, 24]. The parotid gland is the most common location (76.56%) followed by submandibular (7.81%) and minor salivary glands (15.63%). The latter sites include buccal mucosa (3 cases), palate (2 cases), retromolar region (2 cases), and nasal septum (1 case) [3, 7, 8, 9]. One case of minor salivary gland origin did not describe a specific site in the oral cavity [10].
Those developing in the major salivary glands typically present with slow- growing mass with or without associated pain. The lesions are usually solitary, but multiple lesions or synchronous association with other benign salivary tumors (pleomorphic adenoma, oncocytoma, Warthin’s tumor, and combined sebaceous lymphadenoma-Warthin’s tumor) can occur [3, 11].
Grossly, SPA is well-circumscribed, firm or rubbery mass with scattered multiple small cysts surrounded by normal salivary gland parenchyma. The lesion can vary in color ranging from white-tan to yellow-white.
Microscopically, the lesion is unencapsulated or partially encapsulated and consists of multiple irregular lobules containing proliferation of ducts and acini in a background of densely fibrotic stroma. The ductal component frequently demonstrates cystically dilated with flattened to cuboidal cells lining. The ductal cells often develop metaplastic change into a variety of cell types including vacuolated, apocrine, foamy, mucous, squamous and sebaceous-like cells. The acinar cells usually contain abundant coarse eosinophilic granules, which are positive for PAS staining and resistant to diastase enzyme. Intraductal epithelial proliferation can occur ranging from minimal to florid hyperplasia forming a solid or cribriform growth pattern. These proliferating epithelial cells can develop variable degree of dysplastic change ranging from mild dysplasia to carcinoma in situ [12, 13, 20]. In addition, atypical hyperplasia of acinic cells may present, which can be mistaken for acinic cell carcinoma. However, lobular architectures of these atypical ducts and acini are preserved. Up-to- date, there has not been a report of invasive carcinoma arising in SPA.
In our present case, we found multiple foci of florid intraductal epithelial hyperplasia. Some of them showed mild cytologic atypia. However, severe dysplastic change that fulfilled the histopathologic criteria for the diagnosis of carcinoma in situ is not observed.
Immunohistochemically, almost entire ductal and acinar structures are surrounded by myoepithelial cell layer, which is immunoreactive with calponin, smooth muscle actin, P63, S-100, and glial fibrillary acidic protein [12, 25]. The ductal and acinar cells are positive for cytokeratin, but negative for CEA, p53, and HER-2/neu. EMA, s-100, and antimitochondrial antibody variably express in ductal cells, while acinar cells with coarse eosinophilic granules react for GCDFP-15 [12, 25]. Estrogen and progesterone receptors positivity have been studied and showed varying results of reactivity [2, 12, 13].
The differential diagnosis of SPA includes polycystic (dysgenetic) disease, chronic sclerosing sialadenitis, and carcinoma, particularly acinic cell carcinoma, mucoepidermoid carcinoma, low-grade cribriform cystadenocarcinoma, and adenocarcinoma (NOS) [3]. The histologic features of polycystic (dysgenetic) disease are characterized by diffusely honeycomb-like cystic dilatation of the ducts without prominent stromal fibrosis and proliferation of ducts and acini. Chronic sclerosing sialadenitis, also known as Kuttner tumor, has prominent fibrosis with varying degree of lymphocytic infiltration. Unlike SPA, it usually has minimal cystic changes with prominent parenchymal atrophy. For excluding malignant neoplasm, the maintenance of lobular architecture, the preservation of myoepithelial layer, and the lack of invasive growth pattern are important clues.
The etiology and pathogenesis of SPA are controversial, initially point to the reactive postinflammatory process. However, regarding the presence of epithelial dysplasia and carcinoma in situ, together with the demonstration of monoclonal-ity of SPA by Skalova et al. [2], there was a debate as to whether SPA represented a neoplastic process. In 2010, Swelam et al. were the first group that found the evidence of EBV viruses in SPA and postulated the role of EBV in the pathogenesis of the lesion [8]. However, the study was limited by the small number of cases (total 3 cases). Despite, their finding of EBV viruses in SPA, there has been no other report that supports their hypothesis.
The treatment of choice for SPA is adequate surgical excision. Although about 30% of the followup cases have recurred, it was generally considered to be the result of inadequate excision or multifo-cality of the lesion than the true recurrence. In our present case, since the patient underwent superficial parotidectomy before mutifocal lesions of SPA developed. Unfortunately, we did not have chance to review the previous specimen. However, we believe that we are coping with the recurrent lesion.
In summary, sclerosing polycystic adenosis is an uncommon tumor-like lesion of the salivary glands with a benign behavior. However, clinically and histologically may be mistaken diagnosed as malignant neoplasm.
REFERENCES
1. Smith BC, Ellis GL, Slater LJ, Foss RD. Sclerosing polycystic adeno-sis of major salivary glands: a clinicopathologic analysis of nine cas-es. Am J Surg Pathol 1996; 20: 161-70.
2. Skalova A, Gnepp DR, Simpson RH, et al. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol 2006; 30: 939-944.
3. Gnepp DR, Wang LJ, Brandwein-Gensler M, Slootweg P, Gill M, Hille J. Sclerosing polycystic adenosis of the salivary gland: A report of 16 cases. Am J Surg Pathol 2006; 30: 154—164.
4. Meer S, Altini M. Sclerosing polycystic adenosis of the buccal mu-cosa. Head Neck Pathol 2008; 2: 31-5.
5. Batsakis J. Sclerosing polycystic adenosis. Newly recognized salivary gland lesion: a form of chronic sialadenitis? Adv Anat Pathol 1996; 3:298—304.
6. Etit D, Pilch BZ, Osgood R, Faquin WC. Fine-needle aspiration biopsy findings in sclerosing polycystic adenosis of the parotid gland. Diagn Cytopathol 2007; 35: 444—447.
7. Noonan VL, Kalmar JR, Allen CM, Gallagher GT, Kabani S. Sclerosing polycystic adenosis of minor salivary glands: report of three cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104: 516— 520.
8. Swelam WM. The pathogenic role of Epstein-Barr virus (EBV) in sclerosing polycystic adenosis. Pathol Res Pract 2010; 206: 565-571.
9. Park IH, Hong SM, Choi H, Chang H, Lee HM. Sclerosing polycystic adenosis of the nasal septum: the risk of misdiagnosis. Clinical and Experimental Otorhinolaryngology 2011
10. Gurgel CA, Freitas VS, Ramos EA, Santos JN. Sclerosing polycystic adenosis of the minor salivary gland: case report. Braz J Otorhinolaryngol 2010; 76: 272.
11. Takyol C, Aktepe F, Hasturk GS, et al. SClerosing polycystic adenosis of the parotid gland presenting with a Warthintumor,. Kulak Buran Bogaz Ihtis Derg 2012; 22(5): 288-292.
12. Skalova A, Michal M, Simpson RH, et al. Sclerosing polycystic adenosis of parotid gland with dysplasia and ductal carcinoma in situ: report of three cases with immunohisto-chemical and ultrastructural examination. Virchows Arch 2002; 440: 29-35.
13. Petersson F, Tan PH, Hwang JS. Sclerosing polycystic adenosis of the parotid gland: report of a bifocal, paucicystic variant with ductal carcinoma in situ and pronounced stromal distortion mimicking invasive carcinoma. Head and Neck Pathol 2011; 5: 188-192
14. Donath K, Seifert G. Sklerosierende polyzys-tische sialadenopathie: Eine seltene nichttumor-ose erkrankung. Pathologe 1997; 18: 368-373.
15. Imamura Y, Morishita T, Kawakami M, et al. Sclerosing polycystic adenosis of the left parotid gland: report of a case with fine needle aspiration cytology. Acta Cytol 2004; 48: 569573.
16. Bharadwaj G, Nawroz I, O’Regan B. Sclerosing polycystic adenosis of the parotid gland. Br J Oral Maxillofac Surg 2007; 45: 74-6.
17. Gupta R, Jain R, Singh S, Gupta K, Kudesia M. Sclerosing polycys-tic adenosis of parotid gland: a cytological diagnostic dilemma. Cy-topathology 2009; 20: 130-2.
18. Martinez VM, et al. Adenosis poliqmstica esclerosante de la glandula parotid. Rev. Otorrinolaringol. Cir. Cabeza Cuello 2009; 69: 41-44.
19. Perottino F, Barnoud R, Ambrun A, et al. Sclerosing polycystic adenosis of the parotid gland: diagnosis and management. Eur Ann Otorhinolaryngol Head Neck Dis 2010; 127: 20-22.
20. Fulciniti F, Losito NS, Ionna F, et al. Sclerosing polycystic adenosis of the parotid gland: report of one case diagnosed by fine-needle cytology with in situ malignant transformation. Diagn Cytopathol 2010; 38: 368-373.
21. Gurgel CA, Freitas VS, Ramos EA, Santos JN. Sclerosing polycystic adenosis of the minor salivary gland: case report. Braz J Otorhinolaryngol 2010; 76: 272.
22. Martinez AB, Juara AM, Fernandez AC. Adenosis poliquistica esclerosante de la glandula submaxilar. Acta Otorrinolaringol Esp 2011.
23. Kim BC, Yang DH, Kim J, Samayoa SR, Na HY, Choi EJ, Kim HJ. Sclerosing polycystic adenosis of the parotid gland. J Craniofac Surg. 2012; 23:e451-452.
24. Mackle T, Mulligan AM, Dervan PA, et al. Sclerosing polycystic sialadenopathy: a rare cause of recurrent tumor of the parotid gland. Arch Otolaryngol Head Neck Surg 2004; 130: 357-360.25.
25. Gnepp DR. Sclerosing polycystic adenosis of the salivary gland: a lesion that may be associated with dysplasia and carcinoma in situ [Commentary]. Adv Anat Pathol 2003; 10: 218-222.


