Frontoethmoidal encephalomeningocele: histopathology and proposed mechanism of developing lesion
Chariyabhorn Khamjai, M.D.
Department of Anatomical Pathology, Maharat Nakhonratchasima Hospital,
Nakhornratchasima 30000, Thailand
E-mail: juneafter@gmail.com
Tumtip Sangruchi, M.D.
Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University
2 Prannok Rd. Bangkok 10700,Thailand
E-mail: tumtip.san@mahidol.ac.th
Tel. + 66 -02- 4196504 FAX + 66 -02- 4114260
ABSTRACT
The purpose of this study is to describe histopathological findings of frontoethmoidal meningoencephalocele (FEEM), and to correlate with the embryogenesis. The study materials were gathered from 86 cases of FEEM, during 1995-2005. The histopathology was observed in terms of the pattern of the lesion, the relation to the skin and surrounding soft tissue, and the differentiation of cell components. The histopathology was described as solid, disperse and reticular patterns of mature neuroepithelium and meningothelial tissue with varying amount of fibrosis. The lesions occupied the dermis, subcutaneous fat and facial muscle. The dermal-epidermal junction was not involved. Regarding histopathology and embryogenesis, misplaced primitive neuroepithelium in the early embryonic stage before development of the epidermis, muscle and bone are the proposed pathogenesis from this study mechanism for developing such lesion.
INTRODUCTION
Frontoethmoidal encephalomeningocele (FEEM) is a congenital anomaly described as prenasal masses, which continue to the intracranial tissue via bony defects in the anterior cranium between the frontal and ethmoidal bones. It is relatively common in the Southern and South-East Asia with reported incidence varies between 1: 3,500 to 1:7,500 in Thailand [1, 2]. In contrast with the western countries, the occipital form is mostly found [1, 3, 4]. Several reports classified FEEM for surgical correction [5, 6, 7]. However, the histo-pathology of FEEM has been mentioned but not described in detail. Herein we describe the histopa-thology of FEEM and propose pathogenesis based on histopathology and embryogenesis.
MATERIAL AND METHODS
The materials were slides of Hematoxylin and Eosin (HE) gathering from surgical specimens of resected FEEM during 1995 to 2005 at the Department of Pathology, Siriraj Hospital. Recurrent cases were excluded from the study. In case that the neuroepithelial tissue was difficult to recognize, additional histochemistry (Masson trichrome, reticulin) was included. In equivocal cases, menin-gothelial cells and astrocytes were confirmed by immunopositive for epithelial membrane antigen (EMA) and glial fibrillary acidic protein (GFAP) respectively.
The histopathology evaluation focused on the pattern of FEEM, the cell components and the relation of FEEM to the skin and surrounding soft tissue.
Sections from 7 cases of normal glabella skin from autopsy subjects whose bodies were donated to Siriraj Hospital (age ranged from 33 weeks gestation to 2 years) were also collected to compare the skin and soft tissue architecture of this region.
RESULTS
There were 86 FEEM cases in this study, 44 patients were male and 42 patients were female. The patient’s ages ranged from 2 months to 23 years. FEEM specimens had 1-11 H&E slides per case. Total H& E slides were 230 slides.
Since the histopathology of FEEM varies from well developed cysts to ill define soft tissue masses, we then described the lesions into 3 categories as follow:
3.1 Solid pattern was defined as a well-demarcated mass or cyst. Within the mass were mature well-organized or disorganized neurons and glial cells. Numerous blood vessels and fibrous tissue encircling the masses were often seen. (Fig 1)
3.2 Disperse pattern was defined as irregular islands of neuroepithelial tissue of variable sizes, dispersing among dense fibrous tissue (scar-like fibrosis). (Fig 2)
3.3 Reticular pattern was defined as streaks or small groups of meningothelial cells or astrocytes admixed with dense fibrous tissue (scarlike fibrosis). This pattern was hardly recognized on HE alone. (Fig 3, 4)
There were 17 cases (19.8%) of solid pattern. Remaining 69 cases (80.2%) were disperse, reticular or mixed of three patterns. (Fig 5)
All lesions were composed of mature cells. Well-organized neuroepithelium appeared as grey matter, white matter, cerebral or cerebellar cortex. Ependymal lining and well-formed choroid plexus were found in only 2 cases (2.3%). In 3 cases
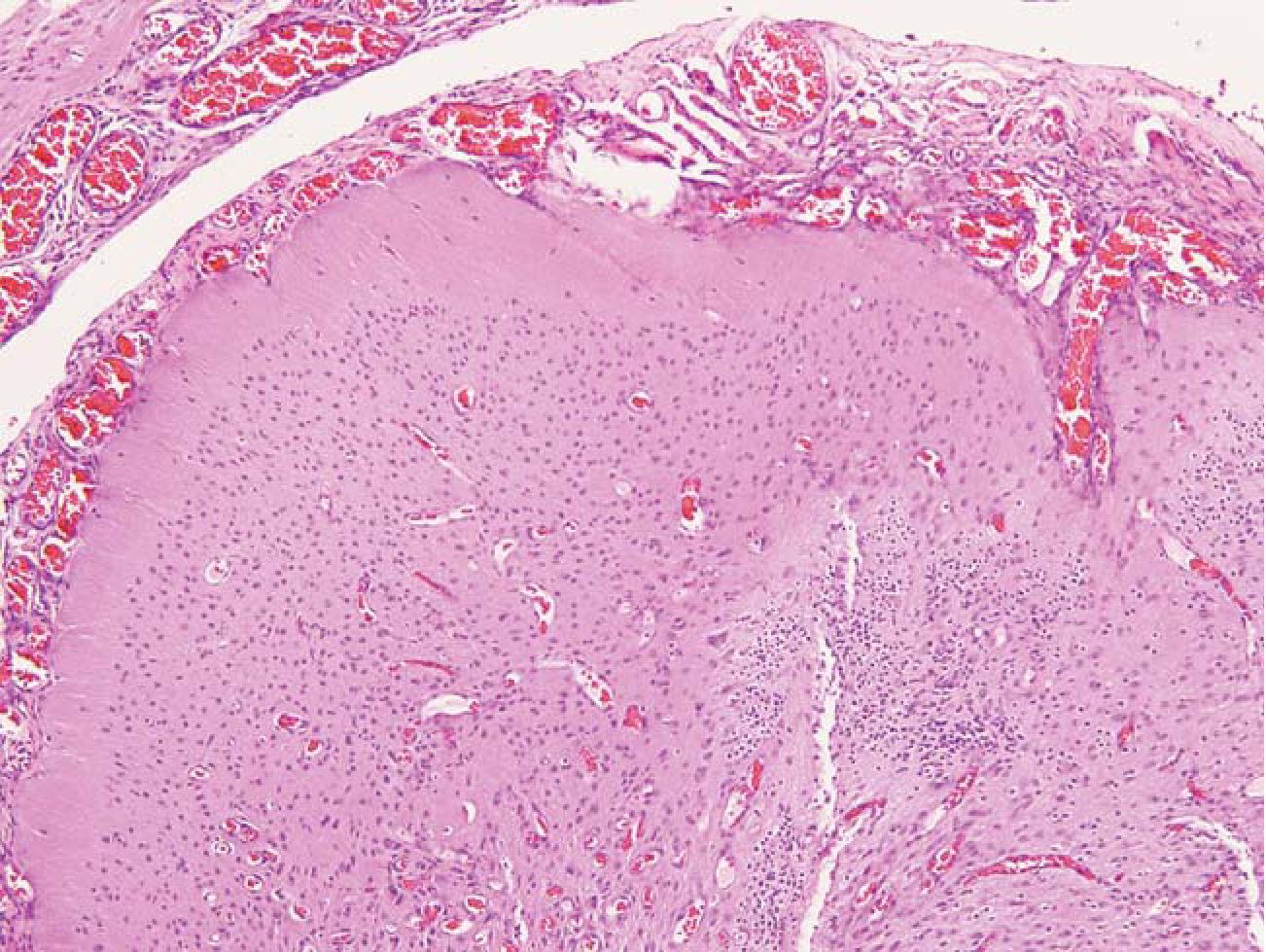
Fig.1 Solid pattern. A well differentiated neuroepi-thelial tissue showed cortical differentiation. The mass was encircled by numerous blood vessels and meningothelial membrane. HE 100x
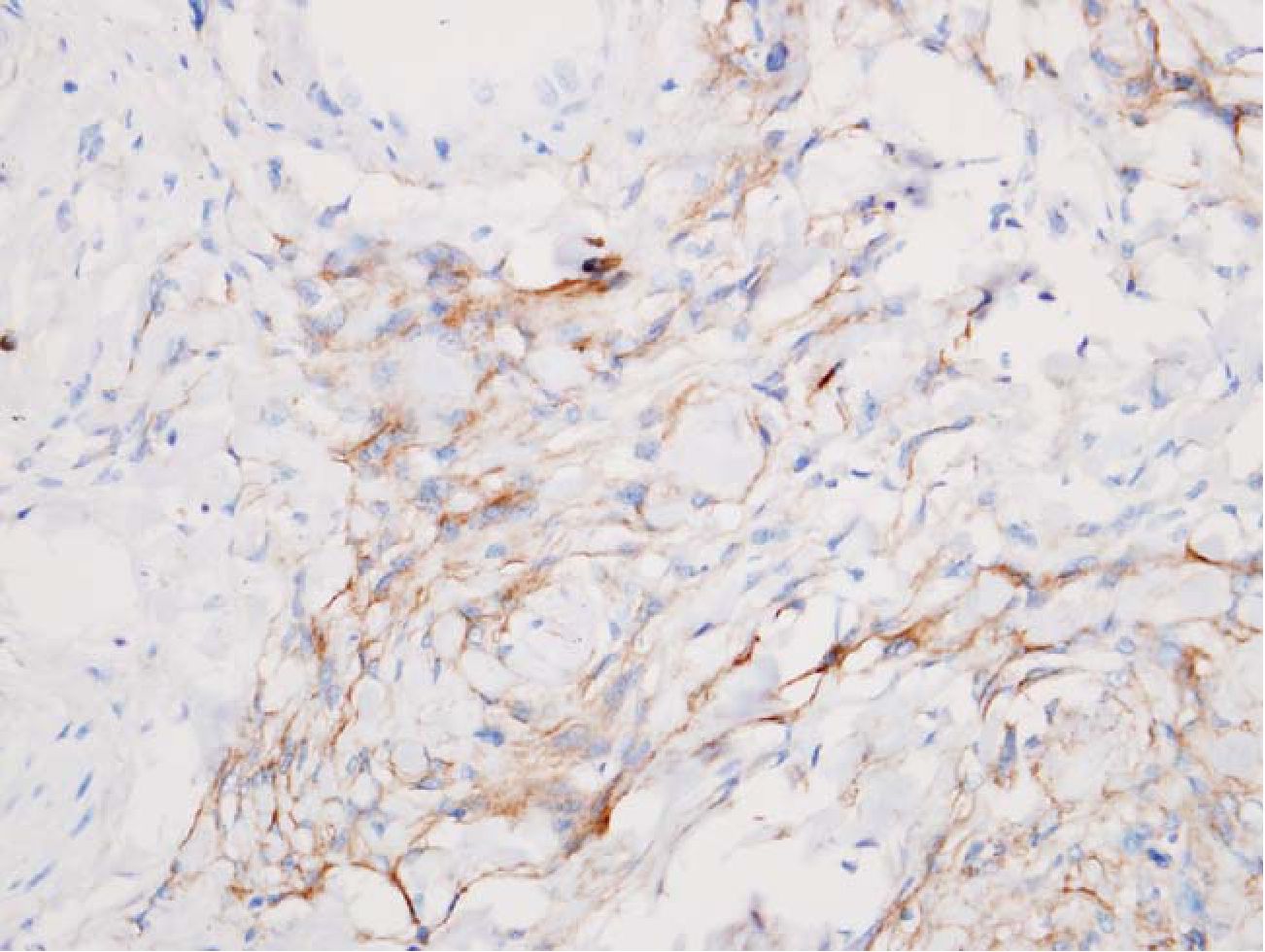
Fig.2 Disperse pattern. A mass comprised interlacing neuroepithelial tissue and dense connective tissue. There is no well- defined membrane around the mass. HE 200x
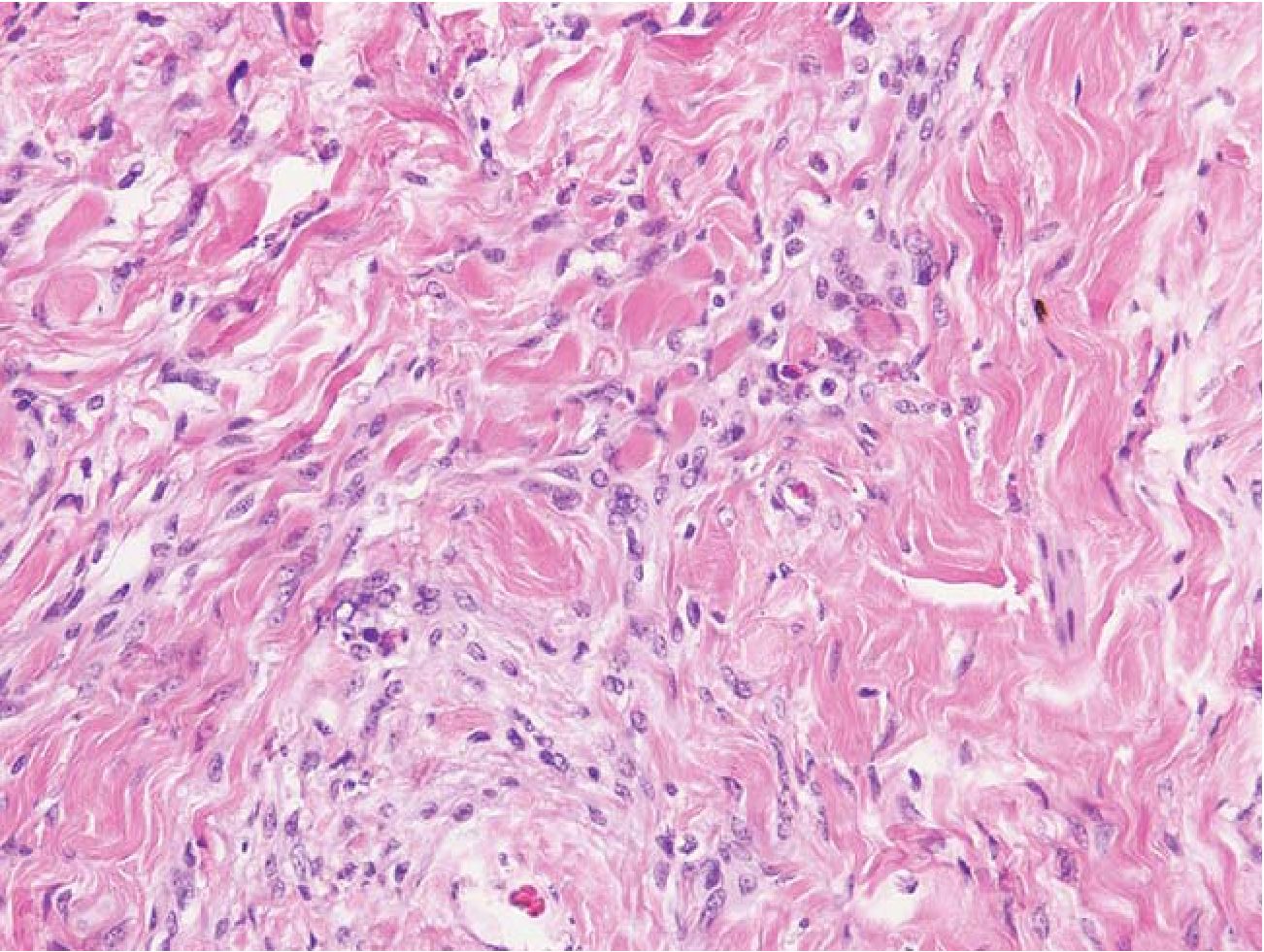
Fig.3 Reticular pattern. An ill-defined mass had streaks of meningothelial cells and astrocytes within densefibrous tissue. HE 400x
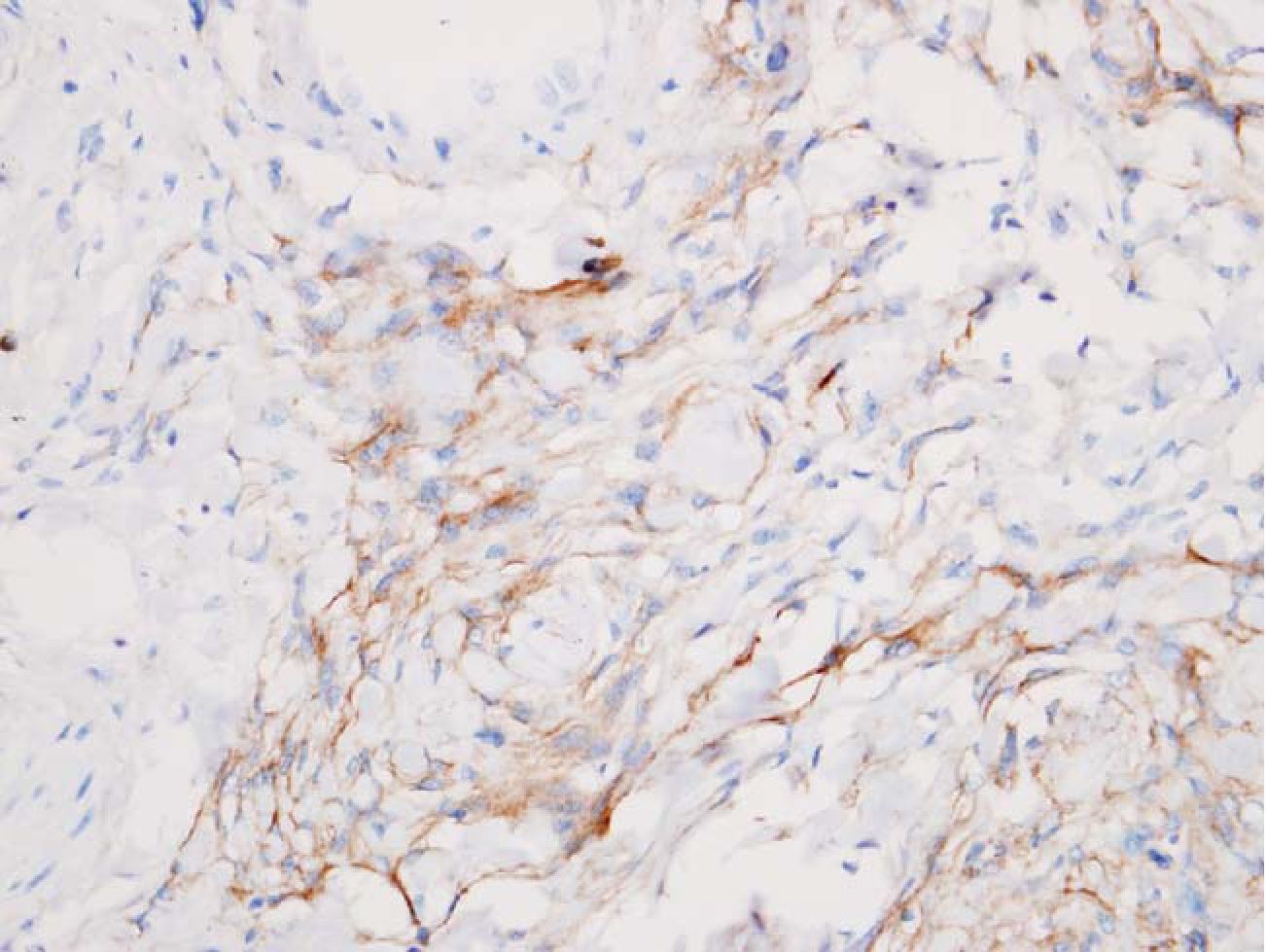
Fig. 4 Meningothelial cells were marked with EMA. HE 400x
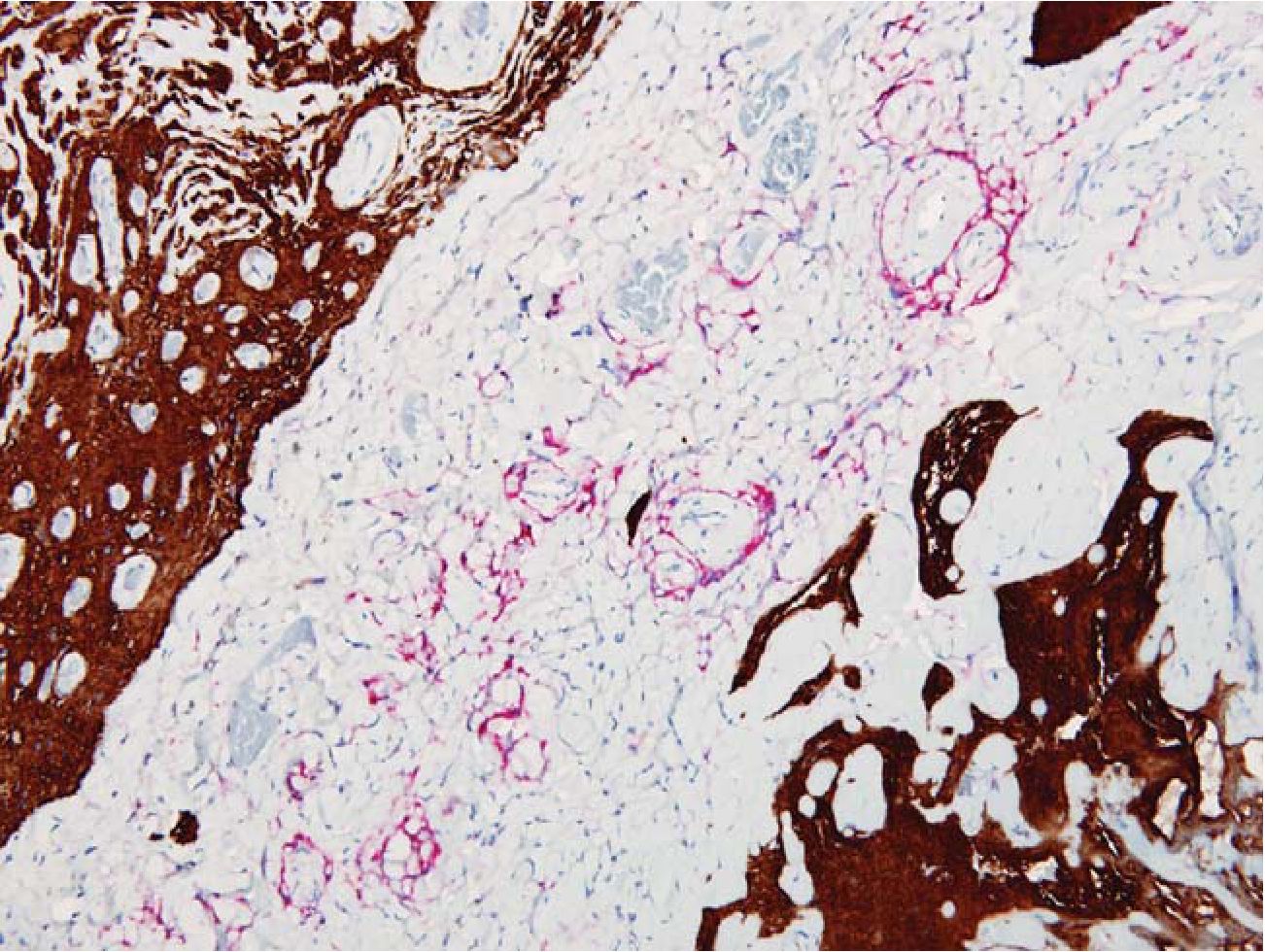
Fig. 5 FEEM in disperse and reticular pattern. Meningothelial cells were marked with EMA (red) while glial tissue were highlighted by GFAP (brown). EMA/GFAP 200x
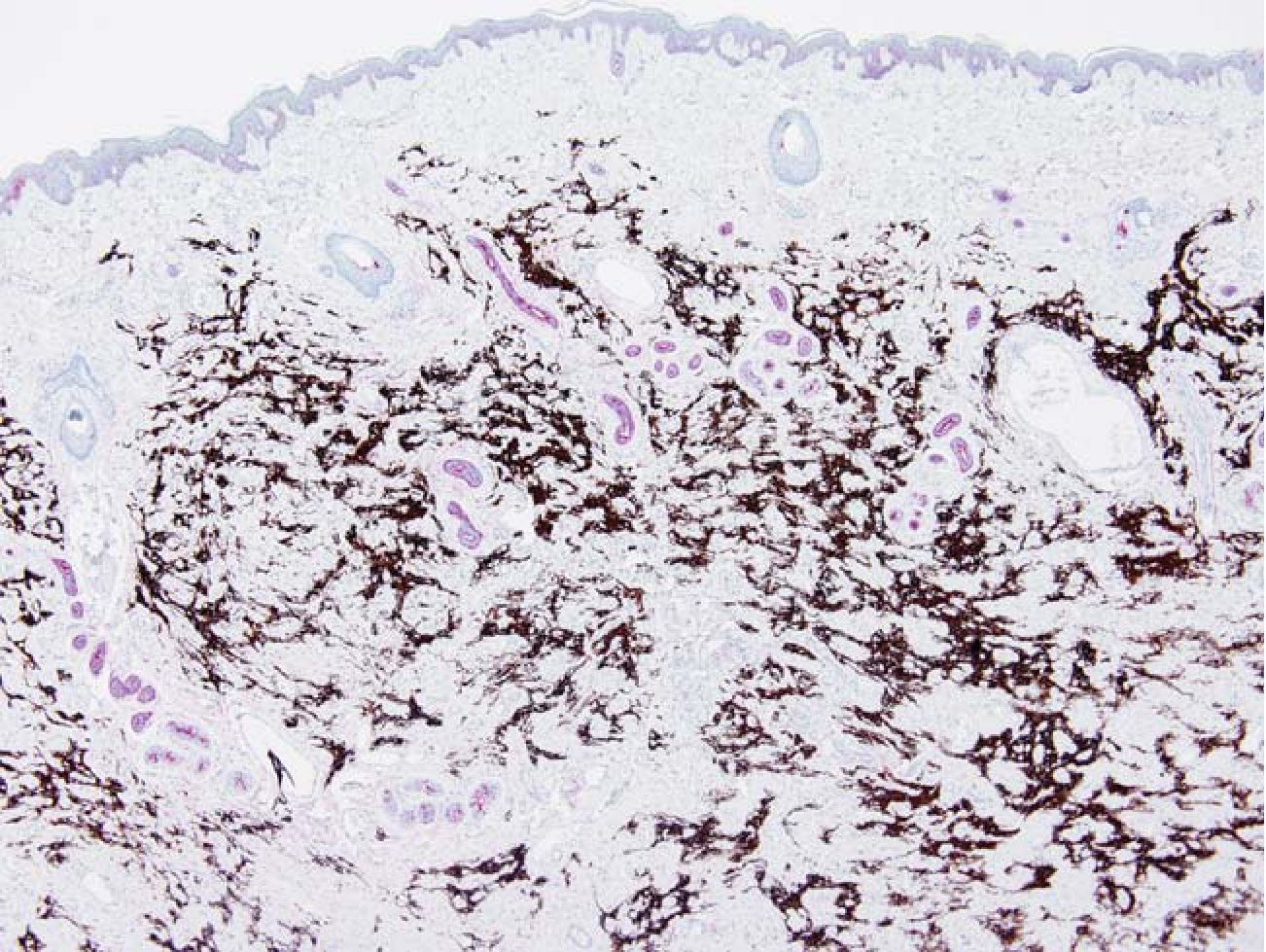
Fig. 6 FEEM in the dermis. The epidermis was thin. The dermal appendages (red) were distorted. Glial tissue (brown). EMA/GFAP 40x
(3.5%), scattered melanocytes were found in the neuroepithelial islands. Disorganized neuroepithelium and glial tissue were more common. Menin-gothelial cells were identified as a well-developed membrane mimicking arachnoid membrane, or scattered groups of cells. Dense fibrous tissue was present in all cases while striated muscle fibers were not seen in the lesions. Unlike immature teratoma, immature neural epithelium or neoplastic tissue was not seen. Few small lymphocytes are noted in some cases.
Relation of FEEM to the skin was identified in 76 cases in which the skin was intact (88.4 %). All lesions regardless of the patterns were confined in the dermis at the level of the skin appendages or below. (Fig 6) FEEM located between the appendages or replaced the appendage structures. None of the lesions reached the dermal-epidermal junction. The epidermis, if not ulcerated, was thin.
DISCUSSION
The histopathological findings of meningocele varied from case to case. Solid pattern was easily detected but it was found in only one fifth of the cases. Most of the cases were disperse and reticular pattern. On H& E they were sometimes difficult to identified. The findings in this study were similar to those found in nasal gliomas and sequestered meningoceles [8-12]. The constant components were meningothelial cells, astrocytes and fibrous tissue. Neither cellular atypia nor primitive neuroepithelium were described in our study or in previous reports. These entities (FEEM, nasal gliomas, and sequested meningoceles) might be examples of heterotopic tissue during development.
When and how did the neural epithelium misplace? This study showed that the heterotopic tissue occupied any level from the lower dermis to the resected margin from the intracranial component. None of them reached the dermal-epidermal junction and the dermal appendages were distorted or pushed aside. Based on embryogenesis of the skin, the surface ectoderm separated from the neuroectoderm at 4 weeks gestation [13]. At 5 weeks gestation, the ectoderm differentiated to form the epidermis. Not until 12 weeks did the epidermis grew downward, forming dermal papillae and the mesoderm differentiated to form dermal appendages and muscle [14]. Previous studies suggested that any disturbance in separation of neural tube and surface ectoderm at 4 weeks gestation might cause residual neural epithelium at the site of final closure of the rostral neuropore [14, 15]. Our findings, particularly the histopathology of FEEM (neuroectoderm) and mesoderm relationship supported this concept. After 12 weeks, when the mesoderm developed, preexisting neuroepithelium then interfered dermal papillae, dermal appendages, striated muscle and bone differentiation.
CONCLUSIONS
In conclusion, the histopathology of FEEM was described. Reticular pattern was hardly recognized and might be missed. From histopathology point of view, defect of neuroepithelial separation in the early embryonic stage leading to heterotopic neuroepithelium are the proposed mechanism of developing this lesion from this study.
ACKNOWLEDGMENTS
This work was supported by Research grants for residents and fellowship and was approved by Siriraj Ethics Committee, Faculty of Medicine, Siriraj Hospital, Mahidol University.
We sincerely thanks Prof. Sriprasit Boon-visut, Plastic surgery unit, Department of Surgery, Faculty of Medicine, Siriraj Hospital, for his helpful advice and Prof. E. W. Hoving, Department of Neurosurgery, University Hospital Groningen, Netherlands, for his reprints.
REFERENCES
1. Suwanwela C, Suwanwela N. A morphology classification of sincipital encephalomenin-gocele. J Neurosurgery 1972; 36: 201-11.
2. Agthong S, Wiwanikit V. Encephalomenin-gocele cases over 10 years on Thailand: a case series. BMC Neurol 2002; 2: 3.
3. Gerbhard F, Chongdee S. Fronto-ethmoidal encephalomeningoceles in the population of northern Thailand. Humangenetik 1970; 11: 1-8.
4. Richards CGM. Frontoethmoidal meningoencephalocele: a common and severe congenital abnormality in South East Asia. Arch Dis Child 1992; 67: 717-9.
5. Boonvisut S, Ladpli S, Sujatanond M, et al. A new technique for the repair and reconstruction of FEEM by medial orbital composite-unit translocation. Br J Plast Surg 2001; 54: 93-101.
6. Boonvisut S, Ladpli S, Sujatanond M, et al. Morphologic study of 120 skull base defects in frontoethmoidal encephalomeningocele. Plast Reconstr Surg 1998; 101: 1784-95.
7. Nond R, David JD, Moore MH, Cole J. Fron-toethmoidal encephalomeningocele: new morphological findings and a new classification. J Cran Surg 2003; 14: 847-58.
8. Alorainy IA. Sequested meningocele of the scalp. Eur J Radiol 2001; 40: 151-3.
9. Bale PM, Hughes L, deSilva M. Sequested meningocele of scalp: extracranial meningeal heteropia. Human Pathology 1990; 21: 115663.
10. Lowe RS, Robinson DW, Ketchum LD, et al. Nasal gliomata. Plas Reconst Surg 1971; 47: 1-5.
11. McDermott MB, Glasner SD, Nielsen PL. Soft tissue gliomatosis: morphologic unity and his-togenetic diversity. Am J Surg Path 1996; 20: 148-55.
12. Tanii T, Hamada T. A variant of encephalomeningocele: heterotopic brain tissue on the scalp. Dermatologica 1984; 169: 354-8.
13. Keith L, Moore TVN, Persuad, eds. The developing hum. 7th ed. China: Saunders, 2003.
14. Hoving EW, Vermeij-Keers C. Frontoethmoi-dal encephaloceles, a study of their pathogenesis. Pediatr Neurosurg 1997; 27: 246-56.
15. Hoving EW, Vermeij-Keers C, Mommaas-Kienhuis AM, Hartwig NG. Separation of neural and surface ectoderm after closure of the rostral neuropore. Anat Embryol (Berl) 1990; 182: 455-63.


