Diagnostic Pitfalls of Prostatic Adenocarcinoma in Biopsy Specimens
Samrerng Ratanarapee, MD.
Department of Pathology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok
Correspondence: Samrerng Ratanarapee, M.D.
Department of Pathology, Faculty of Medicine Sirriaj Hospital, Mahidol University, Bangkok 10700, Thailand.
Tel. +66-02-411-2005, Fax. +66-02-411-4260
E-mail: samrerng.rat@mahidol.ac.th
ABSTRACT
Prostatic adenocarcioma is a very common male cancer worldwide. Biopsy remains the most reliable means in making definite diagnosis. In the prostate-specific antigen (PSA) screening era, the rate of prostate biopsies rapidly increases, resulting in early detection of very small tumors. In the same time, general pathologists are forced to face more and more biopsy specimens. Many benign mimickers of prostatic carcinoma exist and cause false-positive diagnosis which, subsequently, may lead to serious clinical, psychological, and medicolegal outcomes. These mimickers might be just benign structures, i.e. rectal tissue, Cowper’s gland, paraganglion, seminal vesicle, and ejaculatory duct; benign pathologic or physiologic changes, i.e. atrophy, hyperplasia, adenosis, and even crowded acini; inflammatory processes; i.e. non-specific prostatitis, granulomatous prostatitis, xanthogranulomatous prostatitis, and malakoplakia; or metaplasia, i.e. mucnous metaplasia. All mentioned mimickers are summarized in this communication to support general pathologists when dealing with prostate needle biopsies.
Keywords: Prostatic carcinoma, needle biopsy, benign mimickers, diagnostic pitfalls
Prostatic adenocarcinoma has become the most common malignant tumor in American men. Approximately 200,000 new cases were diagnosed in 2008.1 This tumor is also very common in Thailand. It has been among the five most common cancers in Thai men for over 20 years.2,3 In Siriraj Hospital, It has been the most common malignant tumor in male for more than 6 years.4
Biopsy remains the only most reliable tool for establishing a definite diagnosis of prostatic carcinoma. In the prostate-specific antigen (PSA) screening era, the rate of prostate needle biopsies substantially increases which has resulted in detection of very small tumors. In the same time, a variety of benign structures or lesions are also obtained. These benign structures or lesions sometimes can simulate prostatic adenocarcinoma and can cause confusion in diagnosis. False-positive diagnosis may lead to serious clinical, psychological, and medico-legal consequences. In this communication the author would like to summarize common benign mimickers of prostatic adenocarcinoma for general pathologists. Common benign mimickers of prostatic adenocarcinoma that may be seen in biopsies are listed in Table 1.
Table 1 Benign mimicker of prostatic adenocarcinoma
Normal structures
Rectal tissue
Cowper’s gland
Paraganglion
Seminal vesicle/ ejaculatory duct
Benign changes
Atrophy
Basal cell hyperplasia
Adenosis (Atypical adenomatous hyperplasia) Crowded acini
Inflammation
Non-specific prostatitis
Granulomatous prostatitis
Xanthogranulomatous prostatitis
Malakoplakia
Metaplasia
Mucinous metaplasia
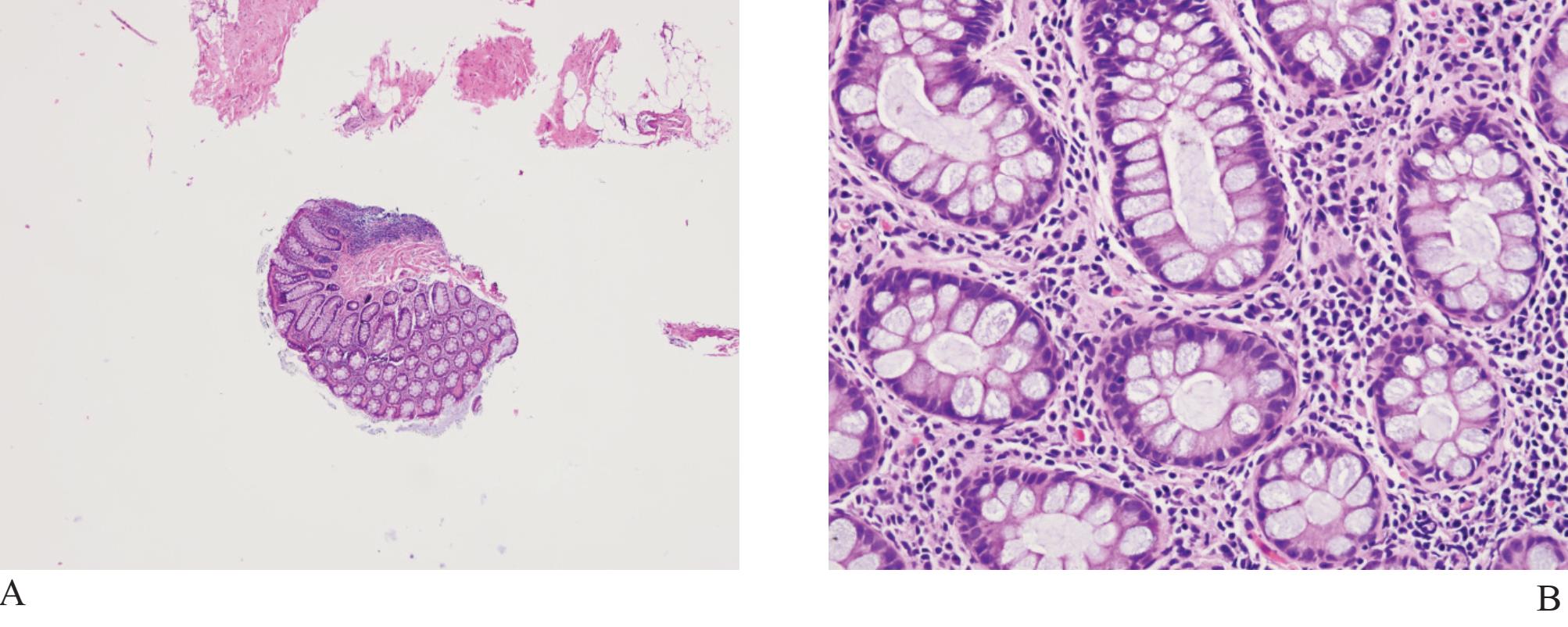
Figure 1
A) Rectal tissue in biopsy. The crypts of Lieberkuhn may look like malignant prostatic acini.
B) Higher power of A) showing goblet cells and lamina propria, not fibromuscular stroma of prostatic tissue
Rectal tissue (Fig 1) is frequently present in transrectal needle biopsy specimens of the prostate gland since it is necessary to pass the biopsy device through rectal canal. These fragments, particularly when distorted, may cause confusion and expert consultation may be required. Schowinsky and Epstein reported 16 needle biopsies of prostate gland with distorted rectal tissue sent for confirmation.5 Blue-tinged intraluminal mucinous secretion, prominent nucleoli, mitotic activity, extracellular mucin, and adenomatous changes were features mimicking prostatic carcinoma. They concluded that the presence of lamina propria, rectal tissue on a detached fragment, associated inflammation, goblet cells, and muscularis propria were useful diagnostic clues in confirming that they were distorted rectal fragments.
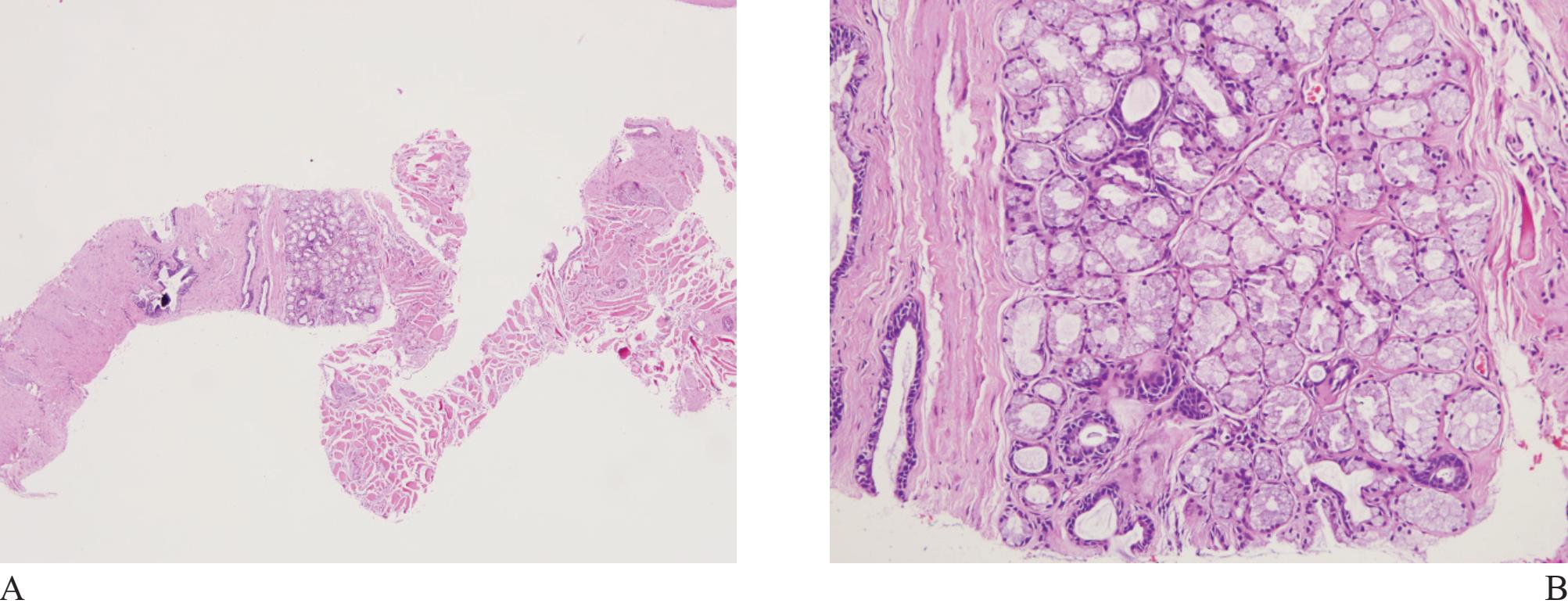
Figure 2 A) Cowper’s gland at low magnification. B) Note compact acini with uniform lining cells, containing abundant cytoplasmic mucin, and benign looking ducts.
Cowper’s glands may be occasionally obtained in prostatic biopsy. They are small, paired bulbomembranous urethral glands that may be mistaken for prostatic carcinoma (Fig 2).6,7 Typically, they are composed ofclosely packed uniform acini lined by benign cells with abundant apical mucinous cytoplasm. Exclusion is based on the fact that the lining cells show negative immunoreactivity for PSA and prostatic alkaline phosphatase.8
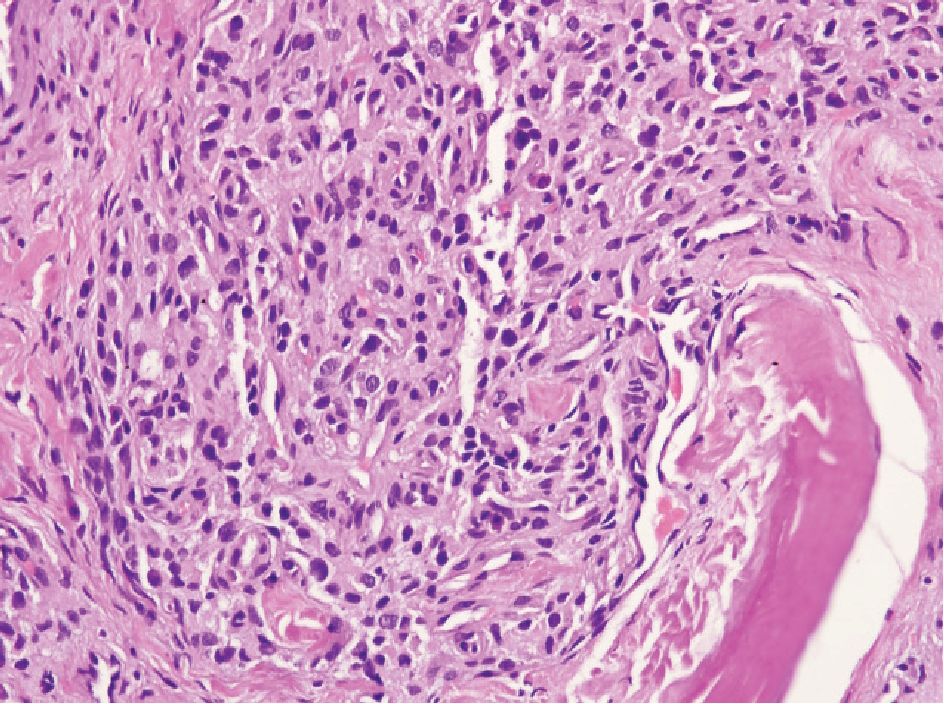
Figure 3 Paraganglion showing small to medium-sized oval or polyhedral cell, arranged in cord-like feature. No nucleoli are present.
Paraganglion can be located within the peripheral prostatic stroma but more commonly in periprostatic tissue. Occasionally, it can be encountered in biopsy specimens and can cause diagnostic problem.9,10 It is characterized by small, solid nests of cells with clear or amphophilic cytoplasm (Fig 3), resembling Gleason pattern 4 prostatic adenocarcinoma. The cells may show hyperchromatic nuclei but nucleoli are not present. In problematic case, immunostain for chromogranin is helpful since they are neuroendocrine cells.
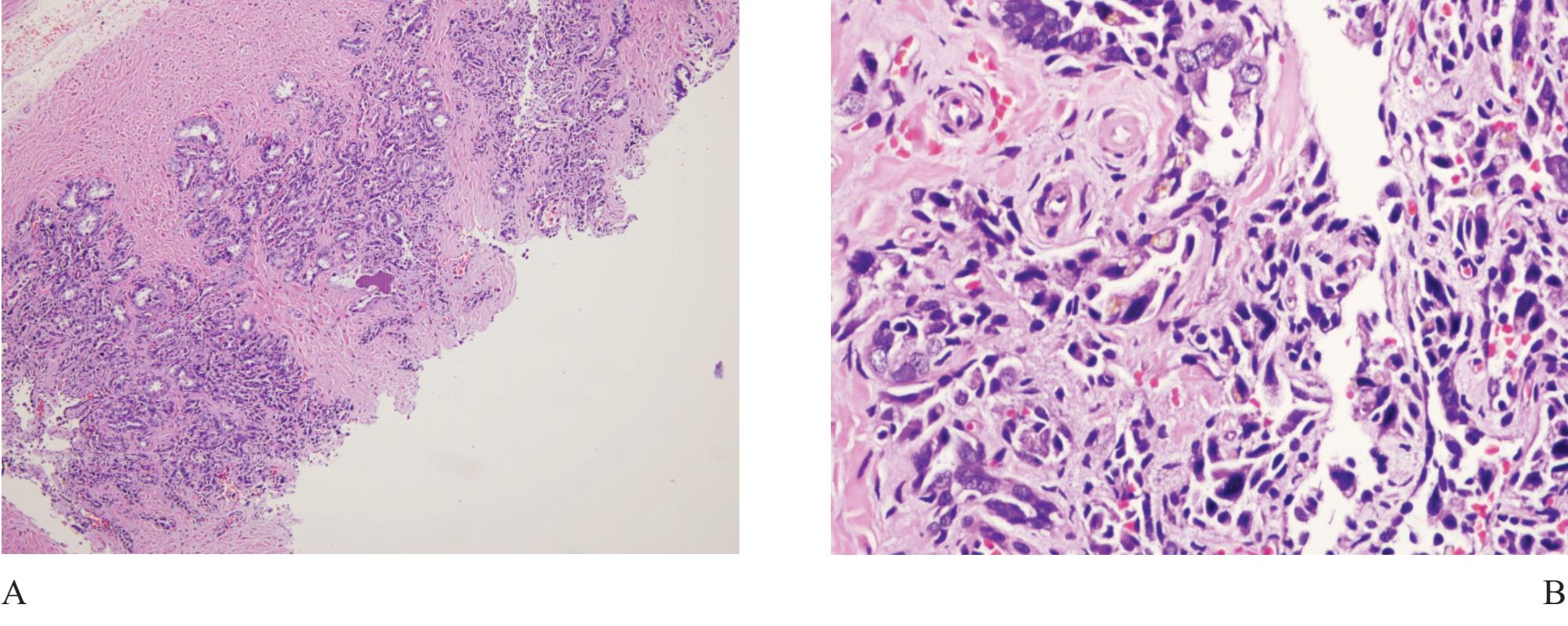
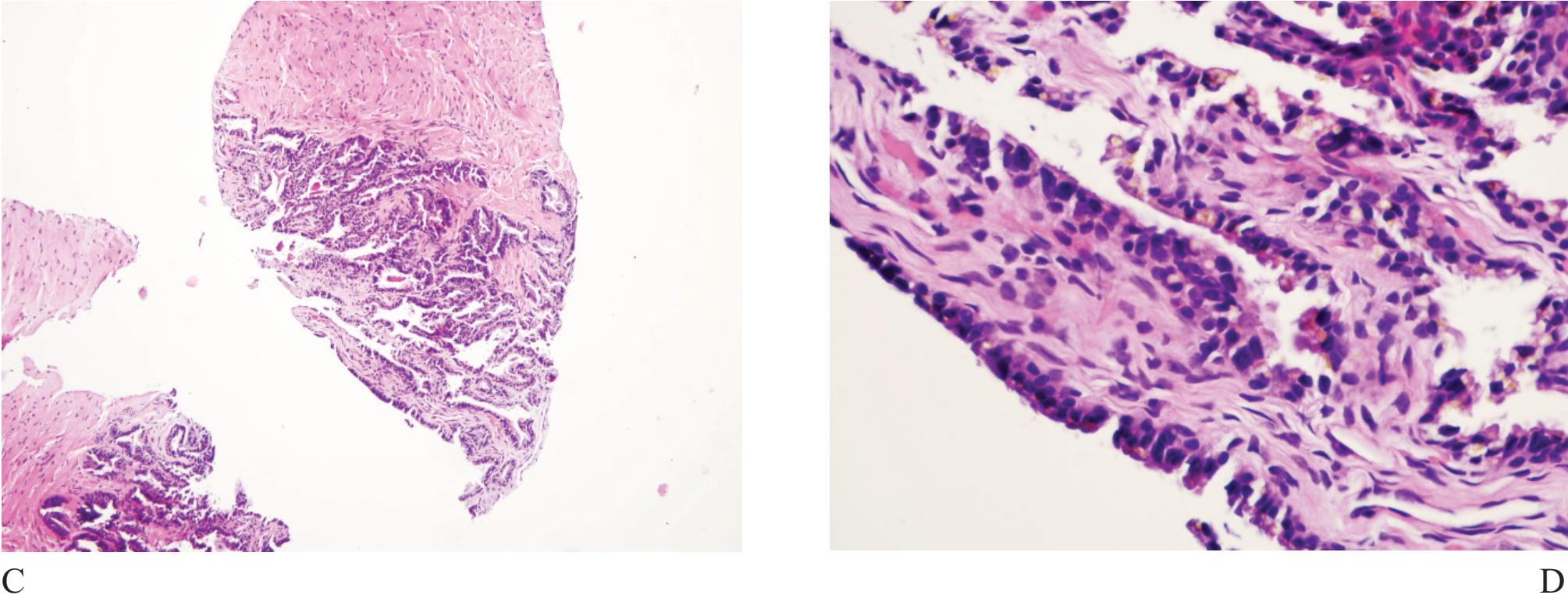
Figure 4 A) Seminal vesicle at low power showing small crowded glands causing confusion in diagnosis. B) Higher power of A). Note exaggerated pleomorphism than conventional atypical appearance in prostatic malignant acinar cells and coarse yellow-brown lipofuscin pigment granules in the cyto plasm. C) and D) Ejaculatory duct with similar lining epithelial cells and pigment.
Seminal vesicle and ejaculatory duct tissue is at times included in needle biopsy specimen. They are composed of numerous small glands and can look like small acinar carcinoma.11 The presence of hyperchromatie nuclei and striking pleomorphism (Fig 4) is of practical importance. Golden-brown lipofuscin pigment is usually identified in the cytoplasm and can strongly support the diagnosis although the same pigment can rarely be found in normal, hyperplastic, preneoplastic (PIN), and malignant prostatic tissue.12,13,14 It is when the cellular atypia and pigmentation are not prominent that a significant diagnostic challenge will occur. Negative prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP) and positive 34PE12 immunostains can be sufficiently confirm that these are seminal vesicular and ejaculatory ductal epithelial cells.15
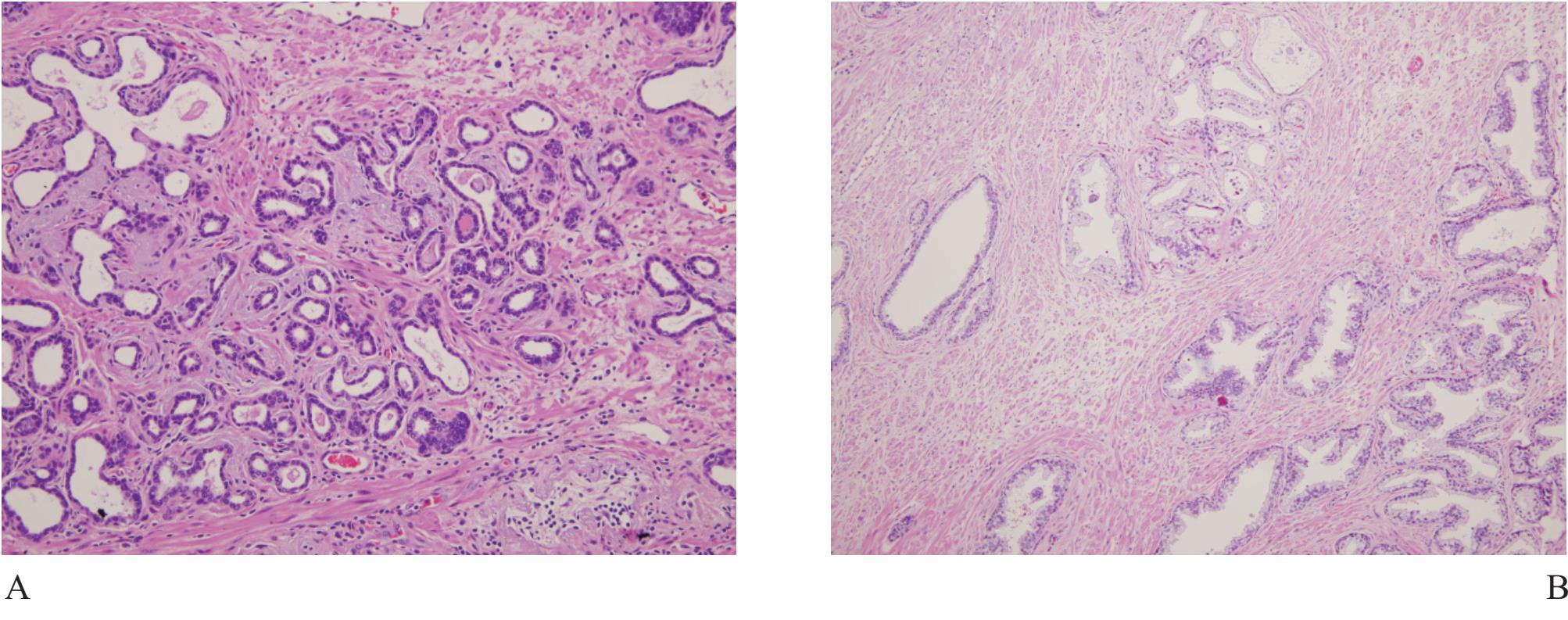
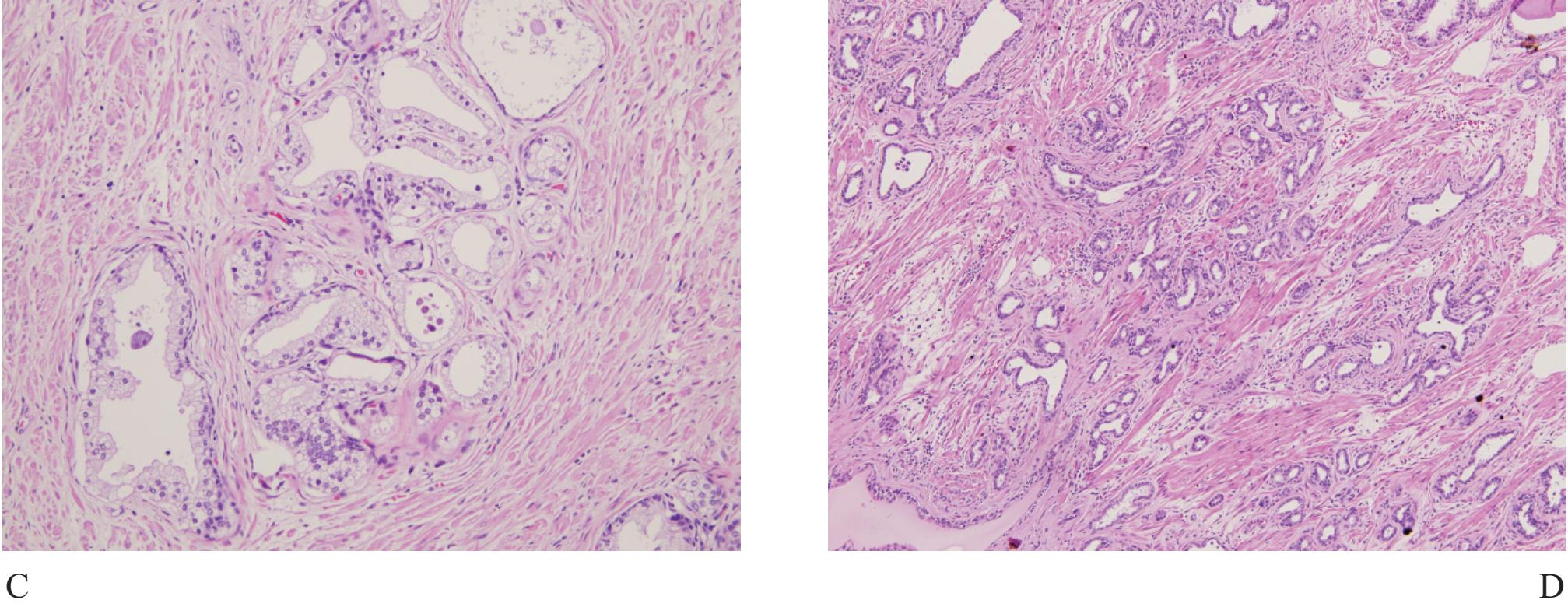
Figure 5 A) Focal atrophic change of prostatic acini simulating Gleason pattern 3 prostatic adenocarcinoma. B) and C) Partial atrophy with clustered acini leading to misdiagnosis. D) Postatrophic hyperplasia, another benign mimicker of prostatic adnocarcioma.
Atrophy is a very common benign change, often seen in the peripheral zone where carcinoma commonly occurs. Therefore atrophic acini are easily obtained at the time of biopsy (Fig 5). Atrophy is a lesion that sometimes is over-interpreted as carcinoma.16 Atrophy is commonly associated with chronic prostatitis. Atrophy maintains a lobular architecture seen in low power with uniform cells and lacks nucleoli. When distorted, it can look very much like small gland carcionoma and immunostain for basal cells should be performed.
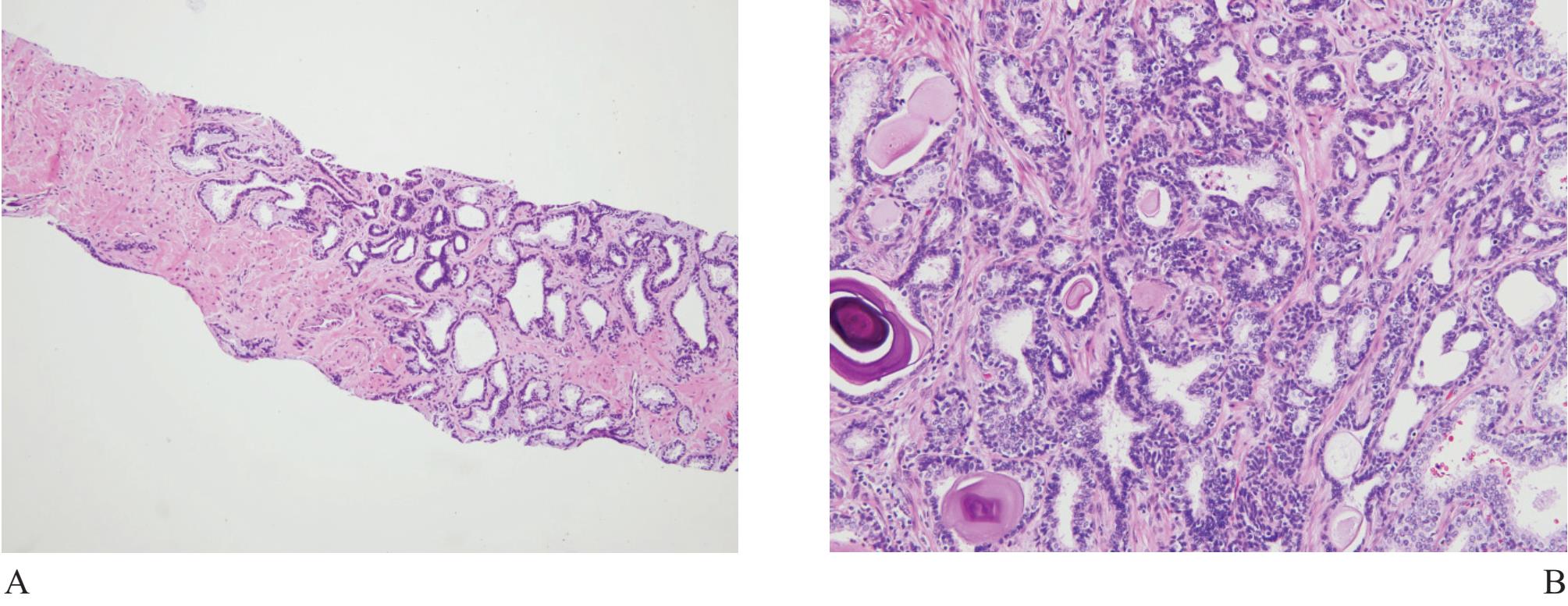
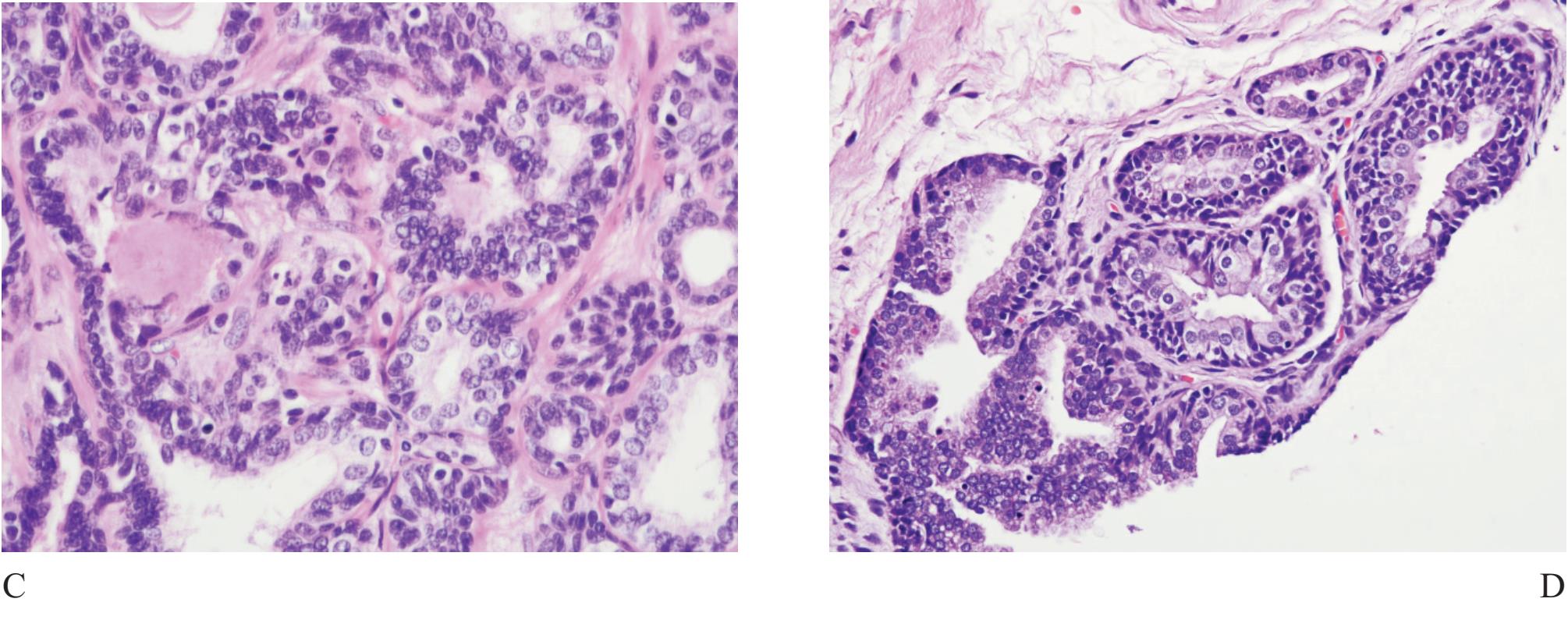
Figure 6 A) Basal cell hyperplasia with crowding looks very much alike prostatic carcinoma. B), C) and D) Higher power showing prominent basal cells with nuclear hyperchromatia and scant secretory cells.
Basal cell hyperplasia (Fig 6) is typically seen as part of nodular hyperplasia which commonly arises in the transition zone,17 but can also affect the peripheral zone.18 It, therefore, can be present in biopsy specimens, causing confusion in diagnosis.
It is characterized by small uniform cells with dark nuclei. Complete basal cell hyperplasia appears in solid nests of dark-blue cells without secretary cell.11 Residual small lumina lined by secretory cells with clear cytoplasm surrounded by multiple layers of basal cells are characteristic features of incomplete form of basal cell hyperplasia. Basal cells have scant cytoplasm and round, oval or spindled hyperchromatic nuclei. Hypercellular stroma as seen in nodular hyperplasia is often present. Basal cell hyperplasia can be separated from small gland carcinoma in most cases but some certain cases require high molecular weight cytokeratin staining.17
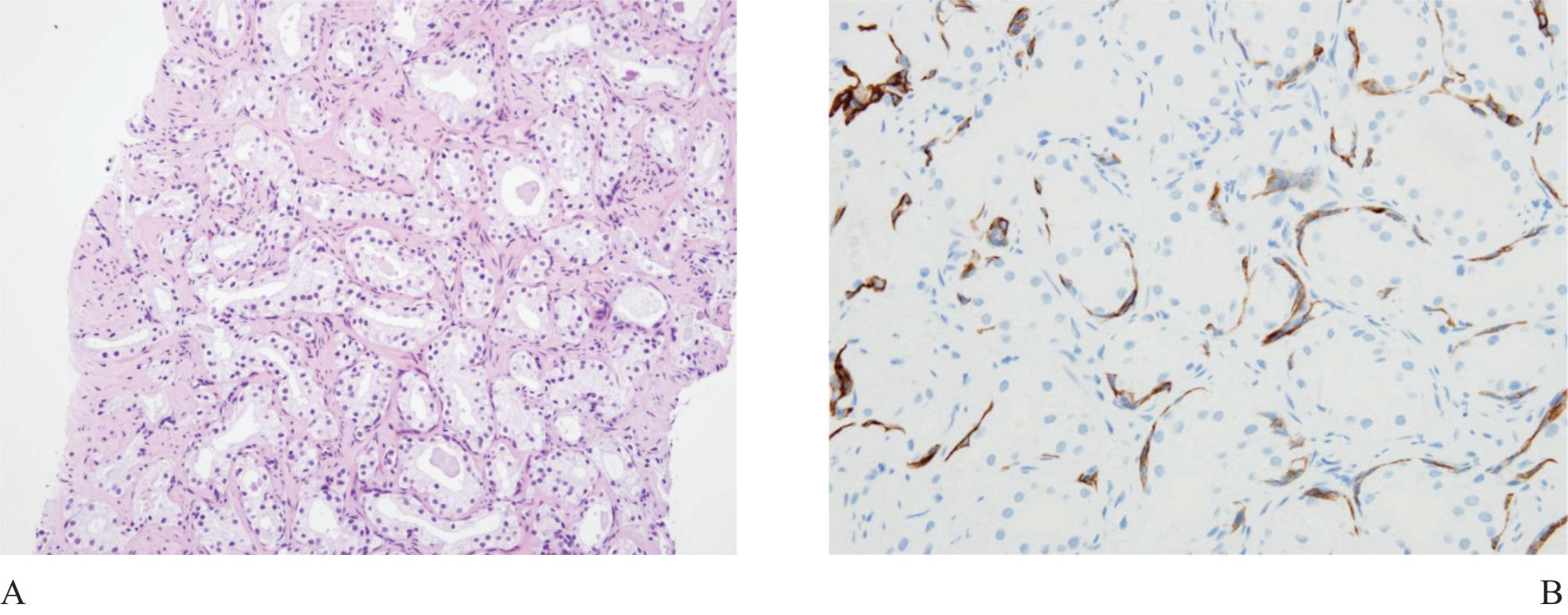
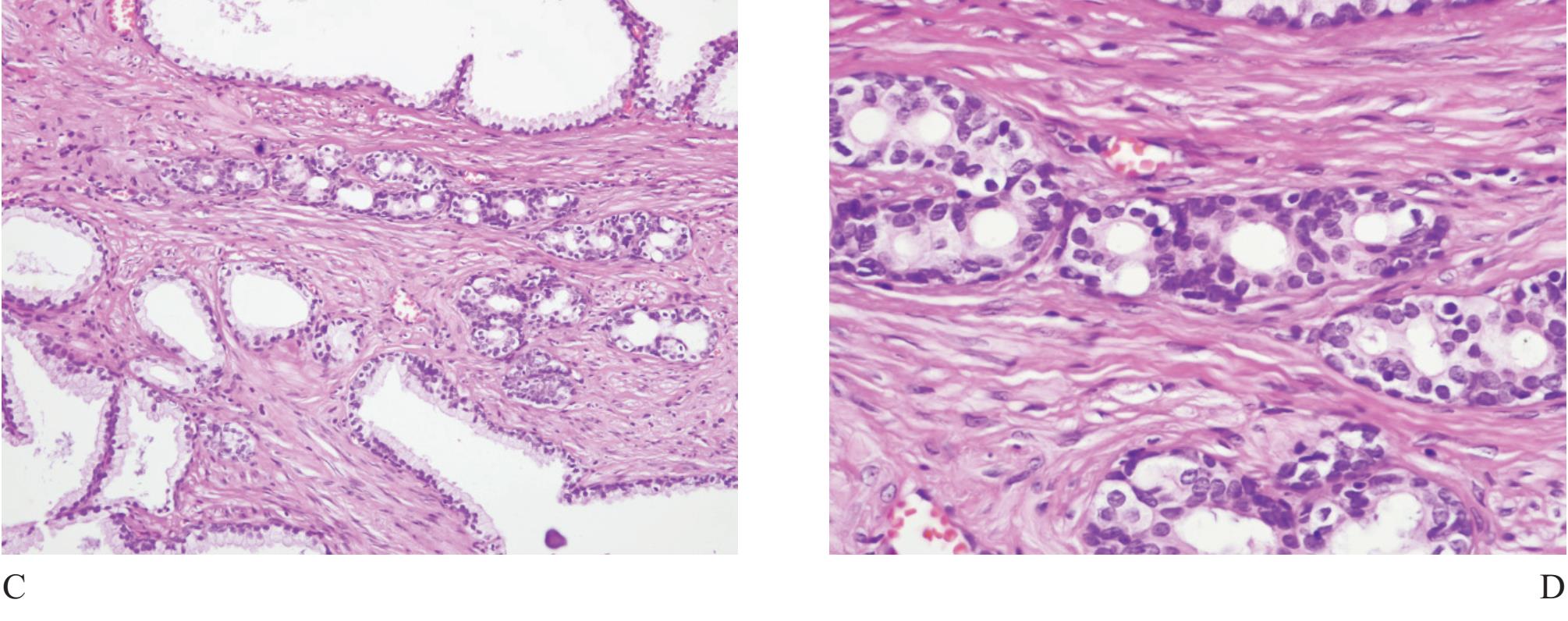
Figure 7 A) Adenosis, the most confusing benign mimicker of prostatic carcinoma. B) High molecular weight keratin (34PE12) staining can highlight the presence of discontinuous basal cells, excluding carcinoma. C) and D) Crowded acini can easily cause false positive diagnosis.
Adenosis (atypical adenomatous hyperplasia) is a well known benign mimicker of prostatic carcinoma, characterized by crowded small acini, formed in well defined nodules (Fig 7). It can simulate small gland carcinoma of the prostate and occasionally expert second opinion is required.19,20,21
The term “crowded acini” is occasionally used by some authors to represent simple focal collection of groups of acini usually with small size, that does not display a nodular feature as adenosis. The presence of basal cells in these lesions excellently confirms their benignancy.
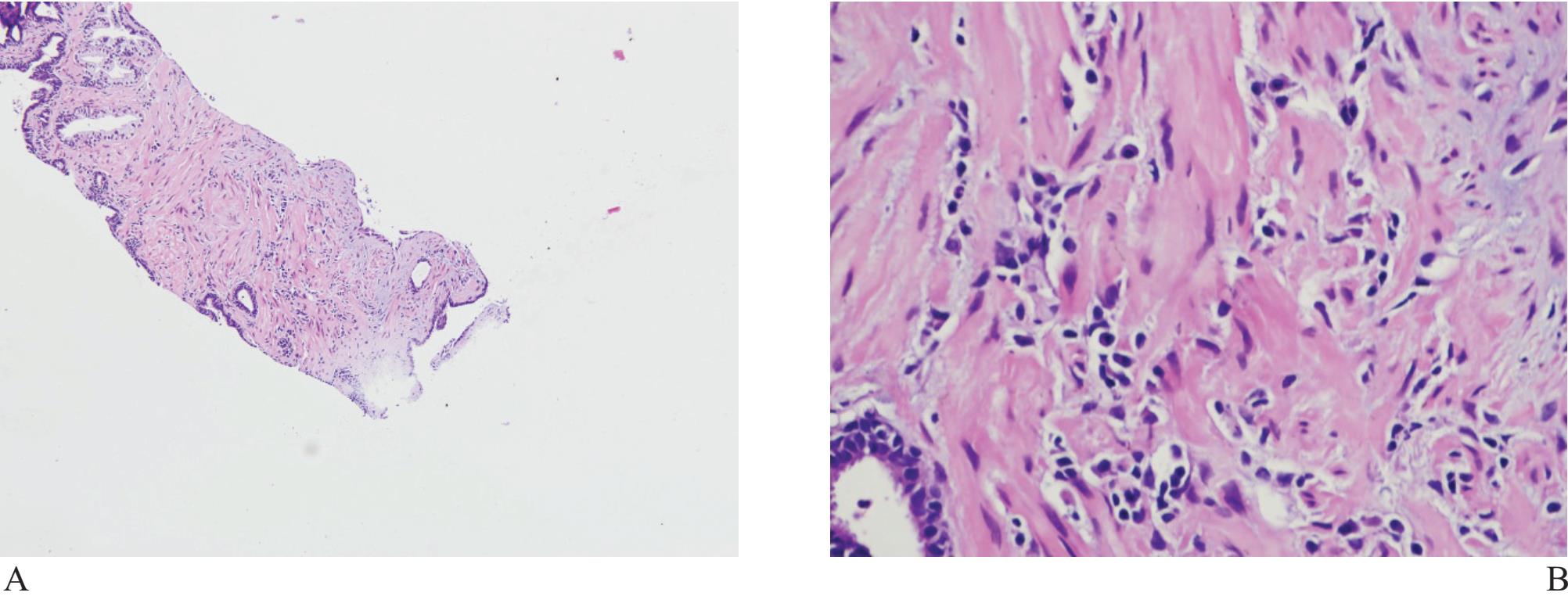
Figure 8 A) and B) Simple non-specific prostatitis associated with artifact may lead to questionable malignancy. Exclusion may require immunohistochemical study.
Inflammatory processes can lead to glandular distortion and nuclear atypia to an extent that they are mistaken for malignant acini. Inlammatory cells and macrophages can, by themselves, look like tumor cells. Non-specific prostatitis, in which lymphocytes and plasma cells are prominent, can resemble poorly differentiated individual tumor cells (Fig 8).7
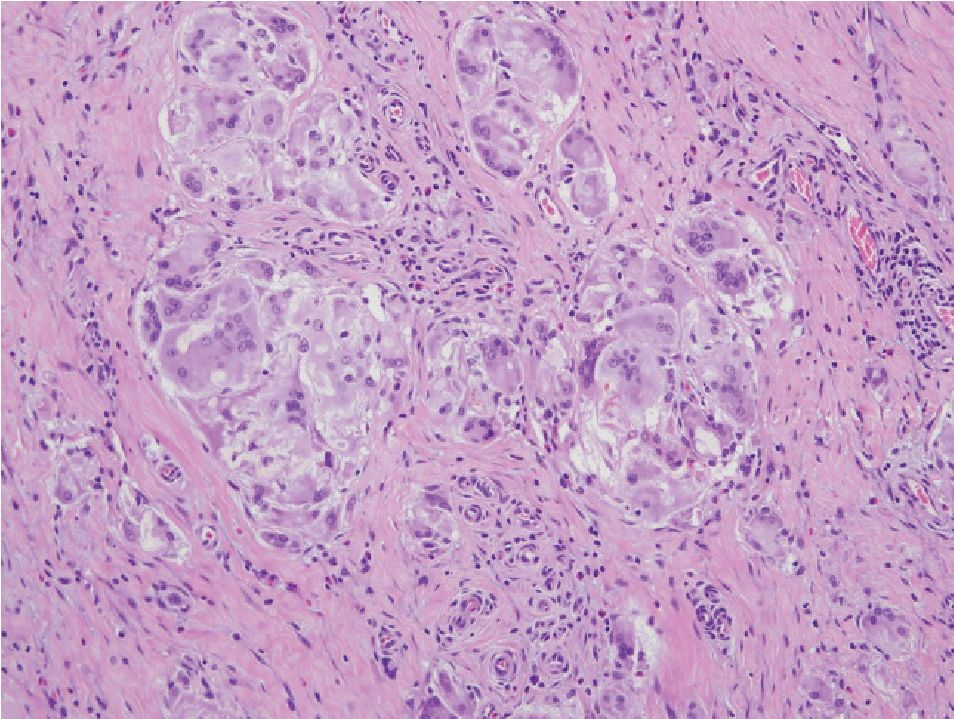
Figure 9 Granulomatous prostatitis showing grouping of macrophages. The presence of other chronic inflam matory cells. Multinucleated giant cells, and, in certain cases, foreign body, will had to recognition of inflammatory process
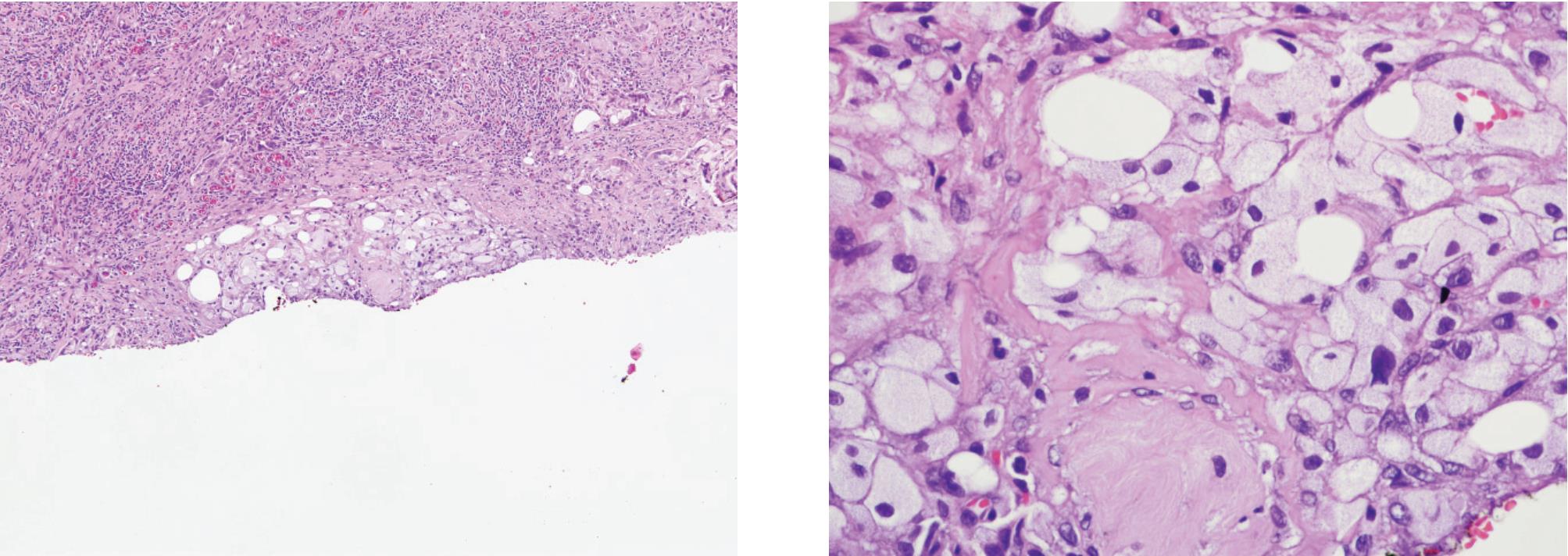
Figure 10 A) and B) Xanthogranulomatous prostatitis showing mainly foamy macrophages but lacking acinar configuration. Epithelial and histiocyte markers can separate these conditions.
Granulomatous prostatitis (Fig 9) may simulate adenocarcinoma.22 This form of prostatitis may be induced by infection, particularly bacterial and fungal, by surgical procedures or may be idio-pathic.23,24 Xanthogranulomatous prostatitis (Fig 10) is defined as a chronic inflammation with aggregation of lipid-laden histiocytes admixed with other chronic inflammatory, cells of the prostate. It may mimic high-grade adenocarcinoma.25
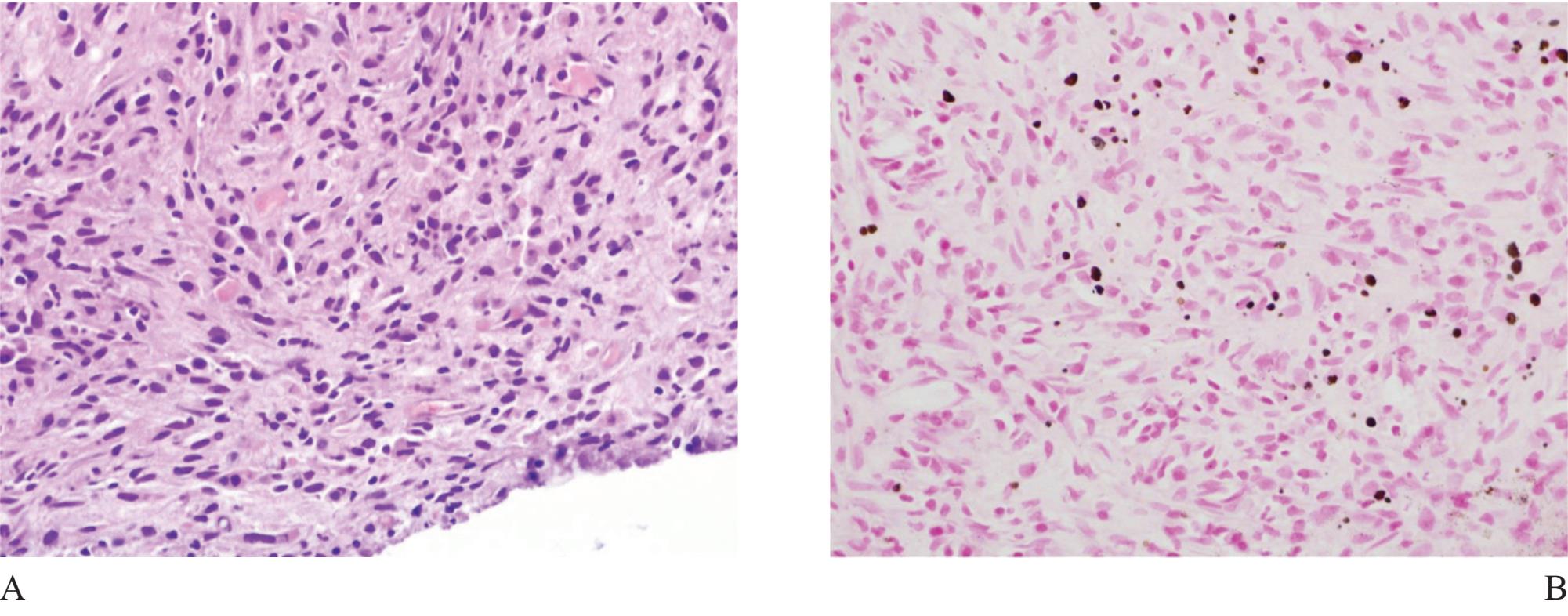
Figure 11 A) Malakoplakia involving the prostate ( H&E). B) Michaelis-Gutmann’s bodies ( Kossa stain) appearing as brown to black spherical bodies are definite for diagnosis.
Malakoplakia is also a granulomatous inflammation associated with bacterial infection, most commonly caused by E.coli, occasionally occurs in the prostate.26 It is characterized by diffuse sheets of histiocytes admixed with other inflammatory cells, mainly chronic (Fig 11). Early lesion may look like carcinoma. Recognition of this lesion requires pathologist’s awareness since confirmation is simple. Demonstration of Michaelis-Gutmann bodies by von Kossa stain, available in most institutes, is final.
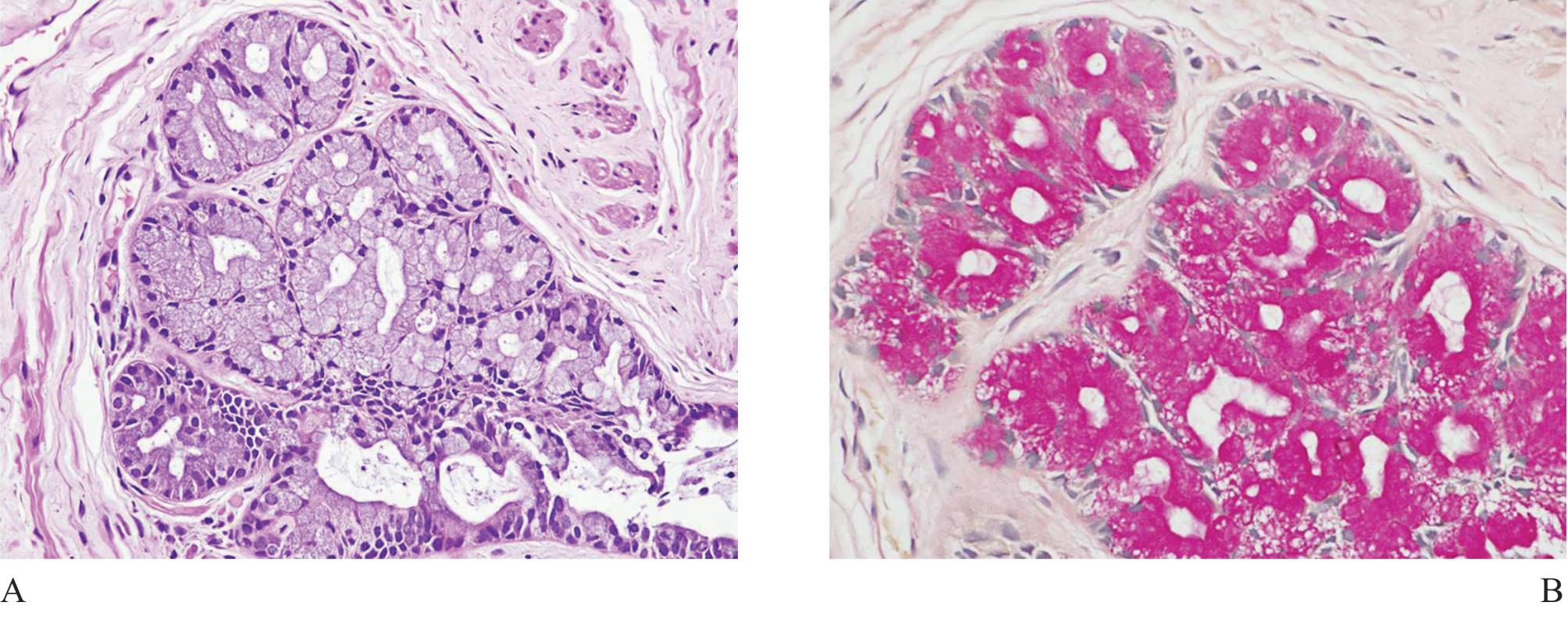
Figure 12 A) Intestinal metaplasia of prostatic acini, resembling Cowper’s gland. No duct is present. B) Intracytoplasmic mucin demonstrated in mucincarmine stain is helpful in diagnosis.
Mucinous metaplasia (Fig 12) is sometimes identified in prostatic biopsy obtained from an atrophic gland.27 It is possible to cause confusion with small acinar carcinoma (the same mechanism that happens to Cowper’s gland). Simple mucicar-mine stain can highlight intracytoplasmic mucin of the acinar metaplastic lining cells, not intraluminal mucin commonly found in prostatic adenocarcino-ma.28
It is important that the surgical pathologists have to pay great attention when facing with prostate needle biopsy. Careful microscopic examination, along with the awareness that many pitfalls exist, will result in a correct diagnosis. They should not hestitate to move to further immunohistochemical study and/or to seek expert consultation since false positive diagnosis, an undesired occurrence, is extremely awful.
REFERENCES
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. Ca Cancer J Clin. 2008; 58:7196.
2. Sriplung H, Sontipong S, Martin N, et al. Cancer in Thailand Vol. III, 1995-1997, Bangkok: Bangkok Medical Publisher;2010.
3. Khuhaprema T, Srivatanakul P, Altasara P, et al. Cancer in Thailand Vol.VI, 2001-2003, Bangkok: Bangkok Medical Publisher;2010.
4. Ratanawichitrasin A, editor. Annual report of tumor registry in Siriraj cancer center. Siriraj Hospital, Bangkok:2008,11p.
5. Schowingsky JT, Epstein JI. Distorted rectal tissue on prostate needle biopsy a mimicker of prostate cancer. Am J Surg Pathol 2006;30:866-870.
6. Chughtai B, Sawas A, O’Mally RL, et al. A neglected gland: a review of Cowper’s gland. Int J Androl 2005;28:74-77.
7. Srigley JR. Benign mimickers of prostatic adenocarcinoma. Mod Pathol 2004;17:328-384.
8. Sabboorian MH, Herffman H, Ashfaq R, et al. Distinguishing Coper’s glands from neoplastic and pseudoneoplastic lesions of prostatic imunohistochemical and ultrastructural studies. Am J Surg Pathol 1997;21:1069-1074.
9. Rode J, Bentley A, Parkinson C. Paraganglial cells of urinary and prostate: potential diagnostic problem. J Clin Pathol 1990;43:13-16.
10. Ostrowski ML, Wheeler TM. Paraganglia ofthe prostate. Location, frequency, and differentiation from prostatic adenocarcinom. Am J Surg Pathol 1994;18:412-420.
11. Srigley JR. Small acinar patternes in the prostate gland with emphasis on atypical adenomatous hyperplasia and small acinar carcinoma. Semin Diagn Pathol 1988;5:254-272.
12. Brennick JB, O’Connell JX, Dickersin GR, et al. Lipofuscin pigmentation (so-called ‘melanosis’) of the prostate. Am J Surg Pathol 1994;18:446-454.
13. Amin MB, Bostwick DG. Pigment in prostatic epithelium and adenocarcinoma: a potential source of diagnostic confustion with seminal vesicular epithelium. Mod Pathol 1996;9:791-795.
14. Leung CS, Srigley JR. Distribution of lipochrome pigment in the prostate gland: biological and diagnostic implications. Hum Pathol 1995;26:1302-1307.
15. Shidham VB, Lindholm PF, Kajdacsy-Balla A, et al. Prostate-specific antigen expression and lipochrome pigment granules in differential diagnosis of prostatic adenocarcinoma versus seminal vesicle-ejaculartory duct epithelium. Arch Pathol Lab Med 1999;123:1093-1097.
16. Oppenheimer JR, Willi ML, Epstein JI. Partial atrophy in prostate needle cores: another diagnostic pitfall for the surgical pathologist. Am J Surg Pathol 1998;22:440-445.
17. Grignon DJ, Ro JY, Ordonez NG, et al. Basal cell hyperplasia, adenoid basal cell tumor, and adenoid cystic carcinoma of the prostate gland: an immunohistochemical study. Hum Pathol 1988;19:1425-1433.
18. Thorson P, Swanson PE, Vollmer RT, et al. Basal cell hyperplasia in the peripheral zone of the prostate. Mod Pathol 2003;16:598-606.
19. Bostwick DG, Srigley JR, Grignon D, et al. Atypical adenomatous hyperplasia of the prostate: morphologic criteria for its distinction from well differentiated carcinoma. Hum Pathol 1993;24:819-832.
20. Bostwick DG, Qian J, Atypical adenomatous hyperplasia of the prostate. Relationship with carcinoma in 217 whole-mount radical prostatictomics. Am J Surg Pathol 1995; 19:506518.
21. Herawi M, Parwani AV, Ivie J, et al. Small g landerlar proliferation on needle biopsies most common benign mimickers of prostatic adenocarcinoma sent in for expert second opinion. Am J Surg Pathol 2005;29:874-880.
22. Oppenheimer JR, kahane H, Epstein JI. Granulomatous prostatitis on needle biopsy. Arch Pathol Lab Med 1997;121:724-729.
23. Mohan H, Bal A, Punia RP, et al. Granulomatous prostatitis - an infrequent diagnosis. Int J Urol 2005;12:474-478.
24. Weidner W, Naber KG. [Prostatitis syndrome consensus of the 6th International Consultation in Paris, 2005] Aktuelle Urol 2006;37:269-271.
25. Chuang AY, Epstein J. Yanthoma of the prostate: amimicker of high-grade prostate adenocarcinoma. Am J Surg Pathol 2007;31:269-271.
26. Sarma HN, Ramesh K, al Fituri O, et al. Mala-koplakia of prostate gland - report of two cases and review of the literature. Scand J Urol Nephrol 1996;30:155-157.
27. Grignon DJ, O’Malley FP. Mucinous metaplasia in the prostate gland. Am J Surg Pathol 1993;17:287-290.
28. Noiwan S, Ratanarapee S. Mucin production in prostatic adenocarcinoma: a retrospective study of 190 redical prostatectomy and/or core biopsy specimens in Department of Pathology, Siriraj Hospital, Mahidol University, Thailand. J Med Assoc Thai 2011;94:224-230.


