Rate of Dyserythropoiesis-like Nuclear Changes in Erythroid Precursors in Bone Marrow
Merisa Klaharn1, Tawatchai Pongpruttipan1, Weerapat Owattanapanich2 and Sanya Sukpanichnant1
Department of Pathology and 2Department of Medicine, Faculty of Medicine Siriraj Hospital,
Mahidol University, Bangkok 10700, Thailand
Correspondence: Sanya Sukpanichnant, MD
Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University
Bangkok 10700, Thailand E-mail: sanya.suk@mahidol.ac.th
Tel: +662 419 6504 Fax: +662 411 4260
• This study was partly presented in the oral presentation by Dr. Merisa Klaharn at the
27th Annual Academic Meeting of the Royal College of Pathologists of Thailand on February 24, 2016 at Ambassador Hotel, Bangkok, Thailand.
• This study was supported by Siriraj Research Fund, Grant Number (10) R015831034,
Faculty of Medicine Siriraj Hospital, Mahidol University.
• Tawatchai Pongpruttipan, Weerapat Owattanapanich and Sanya Sukpanichnant are supported by Chalermphrakiat Grant,
Faculty of Medicine Siriraj Hospital, Mahidol University.
Received 29 March 2016; Accepted 5 May 2016
ABSTRACT
Background: Dyserythropoiesis-like post-mortem changes in erythroid precursors at autopsy can look similar to certain kinds of pathologic processes. Objective: This study is to evaluate nuclear changes in erythroid precursors in marrow aspirate smears and histologic sections at different intervals after taken out from the body, simulating post-mortem changes. Materials and Methods: Marrow aspirate and clotted marrow samples from patients were evaluated for the percentages of erythroid precursors with dyserythropoiesis-like nuclear changes (DLNCs) at 0, 1, 2, 4, 8, 12 and 24 hours after delayed fixation at room temperature. Significant DLNCs were determined at 10% cut-off. Results: Thirteen cases had sufficient samples for evaluation. Significant DLNCs were initially observed at 8 hours in 4 cases (30.8%), at 12 hours in 1 case (7.7%) and at 24 hours in 8 cases (61.5%) after delayed fixation. Conclusion: DLNCs occur in all cases after delayed fixation; the earliest significant one at Hour 8. This phenomenon can interfere with the evaluation of erythroid changes concerning any disease related to dyserythropoiesis when encountered with delayed fixation or as equivalent to post-mortem changes at autopsy.
Keywords: Post-mortem, Bone marrow, Marrow, Dyserythropoiesis Running title: Dyserythropoiesis-like Nuclear Changes in Marrow
INTRODUCTION
Postmortem changes in human body have been inevitably observed by pathologists when autopsy examination is performed. Knowledge about these changes is necessary since it keeps pathologists aware of their interpretation. Many findings found in postmortem changes can look similar to the results of certain kinds of pathologic process. Clinicopathological correlation is important before giving any definite diagnosis, especially when it is an incidental finding of which the patient has never had that underlying disease documented before. Dyserythropoiesis-like postmortem changes in erythroid precursors have been observed as well. [14] These changes include abnormal nuclear shape and budding, cloverleaf-like nuclei or pear-like configuration. In 1964, Hoffman et al. [1] published “rate of cellular autolysis in postmortem bone marrow” about dyserythropoiesis-like change of eryth-roid precursors in postmortem bone marrow which could be seen roughly starting from 3 to 10 hours. Later, in 1977, Findlay [2] published “bone marrow changes in the post mortem interval” about progressive autolytic pattern of marrow cells as well. More recently, in 2014, Chisholm and Heerema-McKen-ney [3] reported about erythroid nuclear irregularities found in fetal and neonatal autopsies that were correlated with clinical features. It showed that they could not exclude the result of fetal erythropoietic response to hypoxic stimuli from a postmortem artifact. These erythroid nuclear irregularities or “dyserythropoietic-like nuclear changes (DLNCs)” were also observed during histologic examination of one fetal autopsy case by two of the authors (MK and SS), leading to the present study. Upon the literature search upon DLNCs, except the study by Hoffman et al., [1] there has not been any other study about the exact changes of erythroid precursors in the same bone marrow at different postmortem intervals. That is the reason why it is difficult to interpret the postmortem changes in bone marrow when myelodysplastic changes are suspected, especially when there is not any underlying history of myelodysplastic changes or myelodysplastic syndrome (MDS). This study was designed to evaluate nuclear changes in erythroid precursors in marrow aspirate smears and histologic sections at different intervals after taken out from the body (delayed fixation) simulating post-mortem changes to determine whether any DLNCs occurred.
MATERIALS AND METHODS
PATIENTS
Marrow aspirate smears and clotted marrow samples from 20 patients of all ages without history of MDS were collected. These samples were leftover specimens from bone marrow examination used for routine work-up or follow-up process at Hematology Unit, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. This study was approved by the Institutional Review Board (IRB) at Faculty of Medicine Siriraj Hospital, Mahidol University [certificate of approval, 140/2557(EC2)] and informed consent was obtained from each patient prior to the collection of the marrow samples.
SPECIMENS
The leftover specimens from bone marrow examination in each patient were processed according to the nature of the marrow samples - marrow aspiration and clotted marrow. One to 3 mL of marrow aspiration samples were anticoagulated with 1.8 mg/mL of potassium EDTA and stored at room temperature (21-25°C). The first smear in each case was performed immediately (Hour 0) after collection of marrow aspiration sample in EDTA and stained with Wright solutions. The same marrow aspiration sample in EDTA in each case was then used to perform the following smears at different intervals of time - 1, 2, 4, 8, 12 and 24 hours, respectively, in the same way as the first marrow aspirate smear. (Figure 1) These marrow aspirate smears were subject for evaluation (see bone marrow morphologic assessment below).
The clotted marrow sample in each case was divided into 7 parts. The first part was fixed immediately in 10% neutral buffered formalin solution, processed, embedded in paraffin, sectioned and stained by hematoxylin & eosin (H&E). The 6 remaining parts of clotted marrow sample in each case were kept separately at room temperature (21-25°C) in a 50 mL centrifuge tube containing 0.9% normal saline to prevent drying artifact. Under this condition, the clotted marrow samples were allowed to follow their natural changes at room temperature, simulating post-mortem change, until the designated time arrived so that the 6 remaining parts of the clotted marrow sample in each case were fixed in 10% neutral buffered formalin solution at 1, 2, 4, 8, 12 and 24 hours after delayed fixation, respectively. Then these sequentially fixed samples of the 6 remaining parts of clotted marrow in each case were then processed, embedded in paraffin, sectioned and stained by H&E, similar to the first part. (Figure 2) Similar to the evaluation of marrow aspirate smears, these clotted marrow sections were subject for evaluation (see bone marrow morphologic assessment below). To enhance the morphologic evaluation of erythroid precursors in clotted marrow sections, periodic acid Schiff (PAS) stain was also performed in each clotted marrow section.
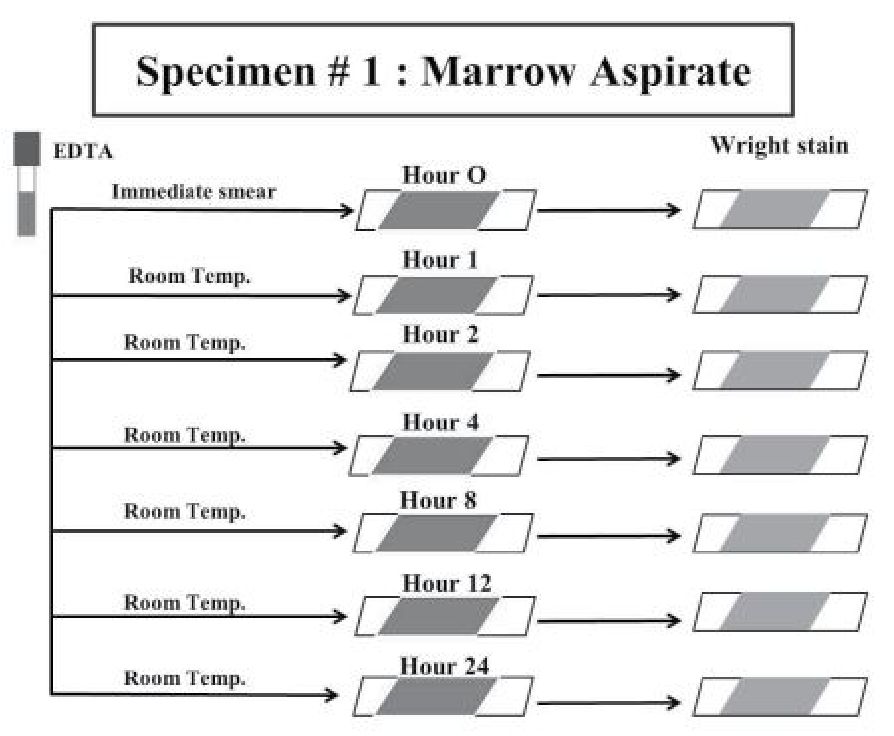
Figure 1 Summary of the handling of leftover specimens from marrow aspirate.
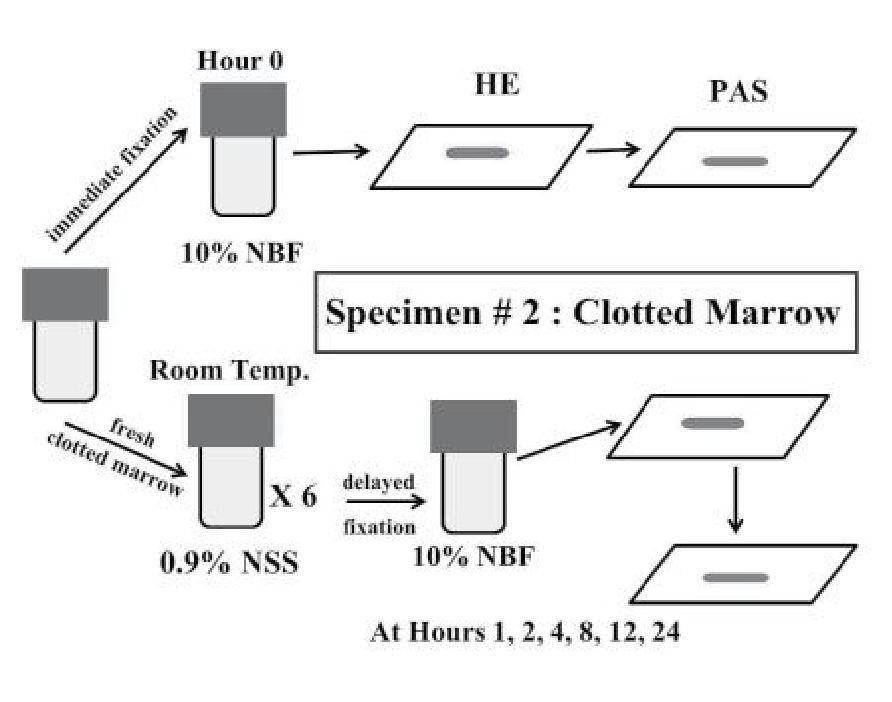
Figure 2 Summary of the handling of leftover specimens from clotted marrow.
BONE MARROW MORPHOLOGIC ASSESSMENT
Light microscopic examination was performed with a 60x and 100x objectives for morphologic features evaluation. Two hundred eryth-roid precursors of all stages (normoblasts) were counted from each slide of marrow aspirate smears and clotted marrow sections. Codes were tagged on each slide to prevent bias during evaluation. The following DLNCs, including abnormal nuclear shape, nuclear budding, cloverleaf-like nuclei and pear-like configuration, were counted and recorded accordingly with agreement upon the morphologic assessment among the 3rd year pathology resident (MK) and the two pathologists (TP, 10 years of experience; SS, 30 years of experience). Significant DLNCs were determined at 10% cut-off according to the WHO classification (2008) upon unilineage dysplasia of >10% of the cells in one myeloid lineage. [5]
RESULTS
Marrow samples were collected from 20 cases but only 13 samples were sufficient for evaluation. They were collected from 5 males and 8 females, ranging from 21 to 72 years of age (mean = 56.2 years; median = 59 years). The underlying diseases in these 13 cases included plasma cell myeloma (5 cases), chronic myelogenous leukemia (2 cases), acute myeloid leukemia (1 case), polycythemia vera (1 case), B-cell chronic lymphocytic leukemia (1 case), small B-cell neoplasm with plasmacytic differentiation (1 case), diffuse large B-cell lymphoma (1 case), and chronic anemia of unknown cause lacking evidence of MDS (1 case).
DLNCs were observed in marrow aspirate smears more easily than clotted marrow sections. However, with the help of PAS stain, DLNCs could be identified with confidence. (Figures 3 & 4) The percentages of DLNCs in all 13 cases at different time intervals were presented in Table 1. At Hour 0, DLNCs could be detected, ranging from 0 to 6% of erythroid precursors. Case 13 was the only case that did not have enough erythroid precursors for evaluation on the marrow aspirate smear at Hour 0 as indicated in the study. But the marrow smear at Hour 1 was sufficient for evaluation and it did not have any DLNCs so that it was still included in the study. Case 3 had the highest percentage of DLNCs (6%) in the marrow smear at Hour 0 but sustained not greater than this percentage in the other 3 following marrow smears at Hours 1, 2 and 4. This case was a treated acute myeloid leukemia (in complete remission) without any documented myelodysplasia related change. The other 12 cases had low percentages of DLNCs at Hour 0 (not greater than 1.5%) and all cases were free from their underlying diseases at the time of marrow sample collection except for 4 cases that still had evidence of marrow involvement by their underlying diseases (cases 1, 2, 4 and 12).
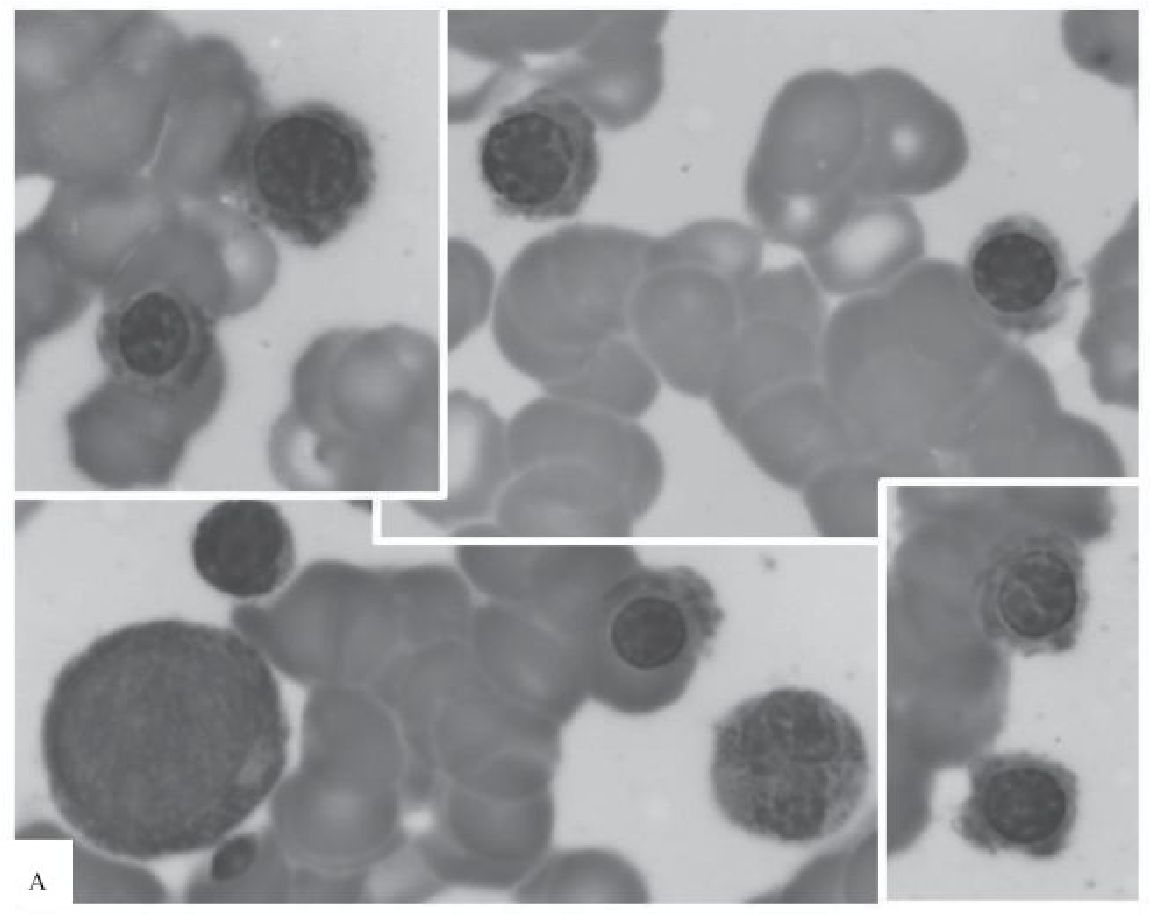
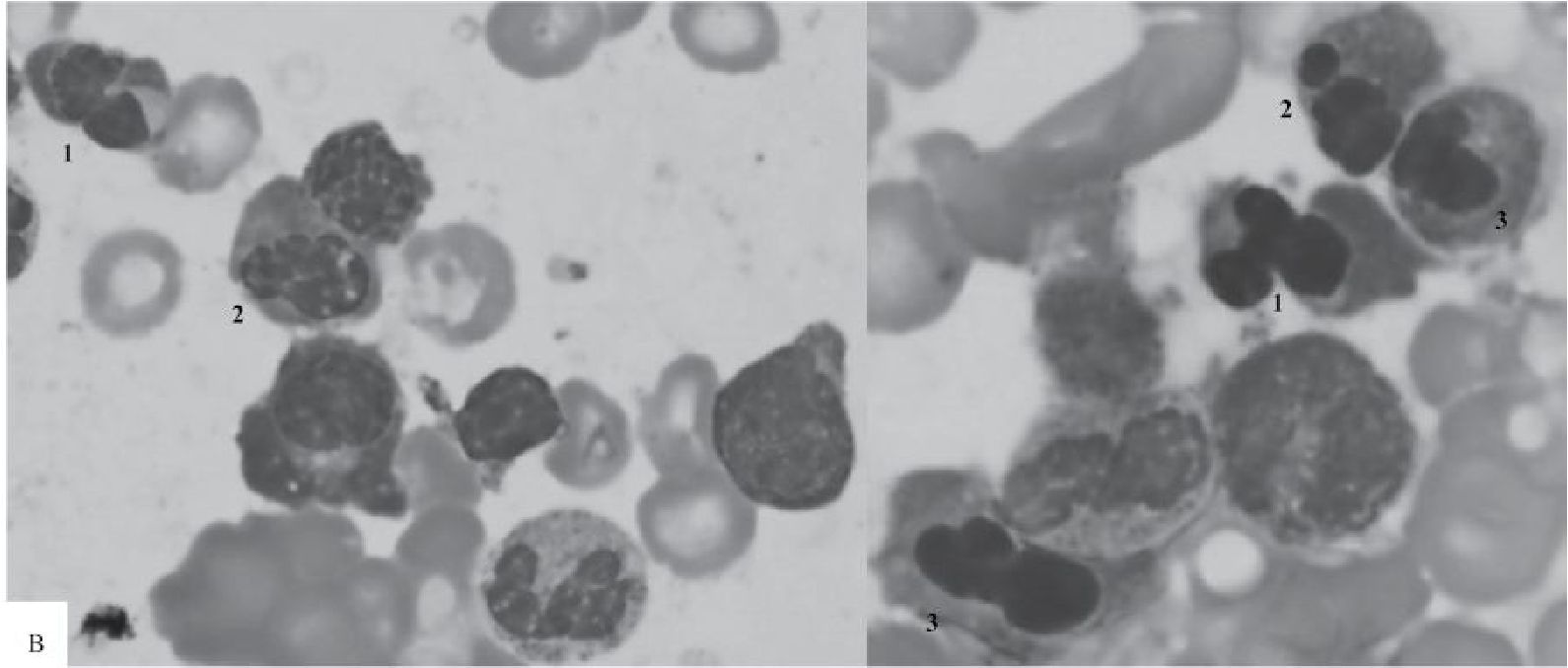
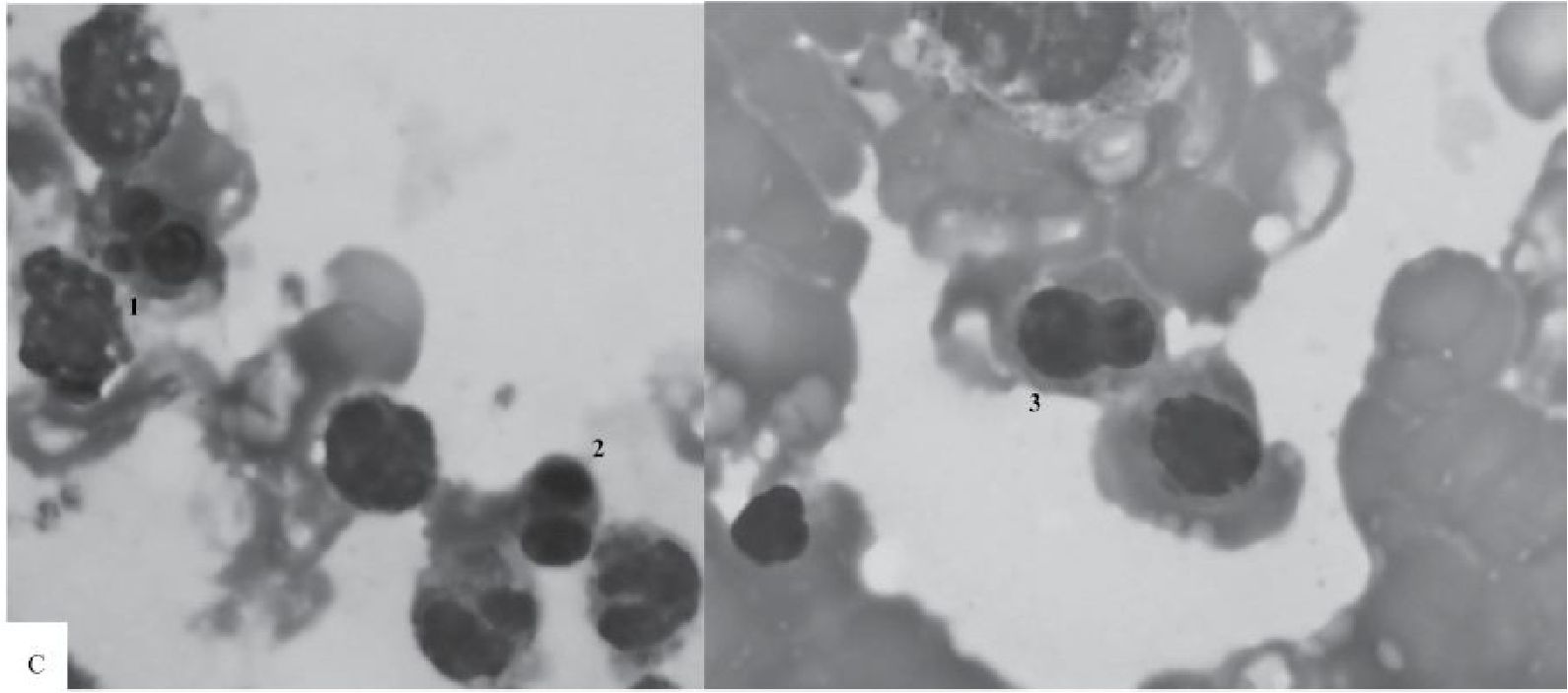
Figure 3 Marrow aspirate smears taken from one patient at 0, 8 and 24 hours after obtaining the marrow sample
A) At Hour 0, a compilation of 4 photomicrographs focusing on various stages of erythroid precursors taken under the same magnification (100x). No nuclear irregularity is observed.
B) At Hour 8, a compilation of 2 photomicrographs shows erythroid precursors with cloverleaflike nuclei (1), nuclear budding (2) and irregular appearance (3) (100x).
C) At Hour 24, a compilation of 2 photomicrographs shows - trilobed (1), bilobed (2), and pear-like (3) nuclei - from left to right (100x).
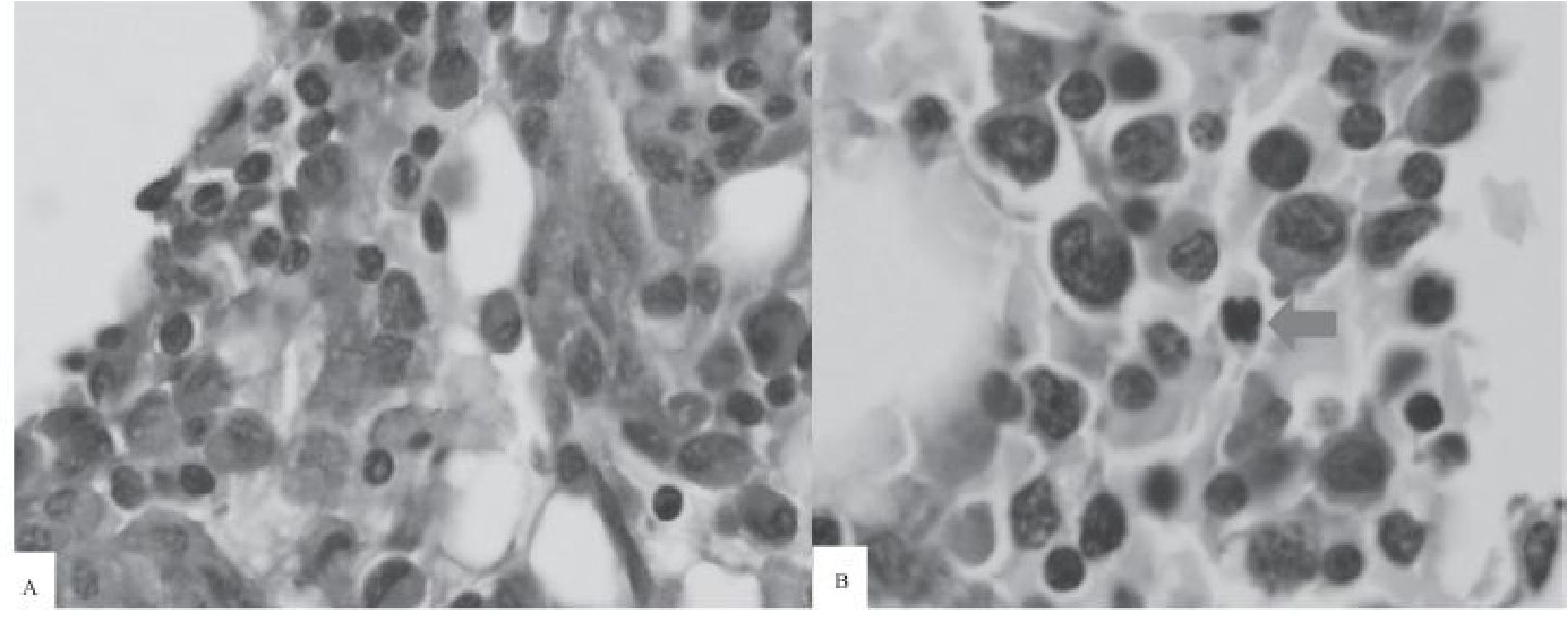
Figure 4 PAS-stained clotted marrow sections taken from another patient at 0 and 8 hours after obtaining the marrow sample.
A) At Hour 0, normal erythroid precursors with pale gray cytoplasm do not show any nuclear irregularity. (100x).
B) At Hour 8, one erythroid percursor shows multilobed nucleus simulating dyserythropoiesis (red arrow) (100x).
Table 1. Dyserythropoiesis-like nuclear changes (DLNCs) at different intervals (shown in percentage of 200 erythroid precursors in marrow aspirate smears)
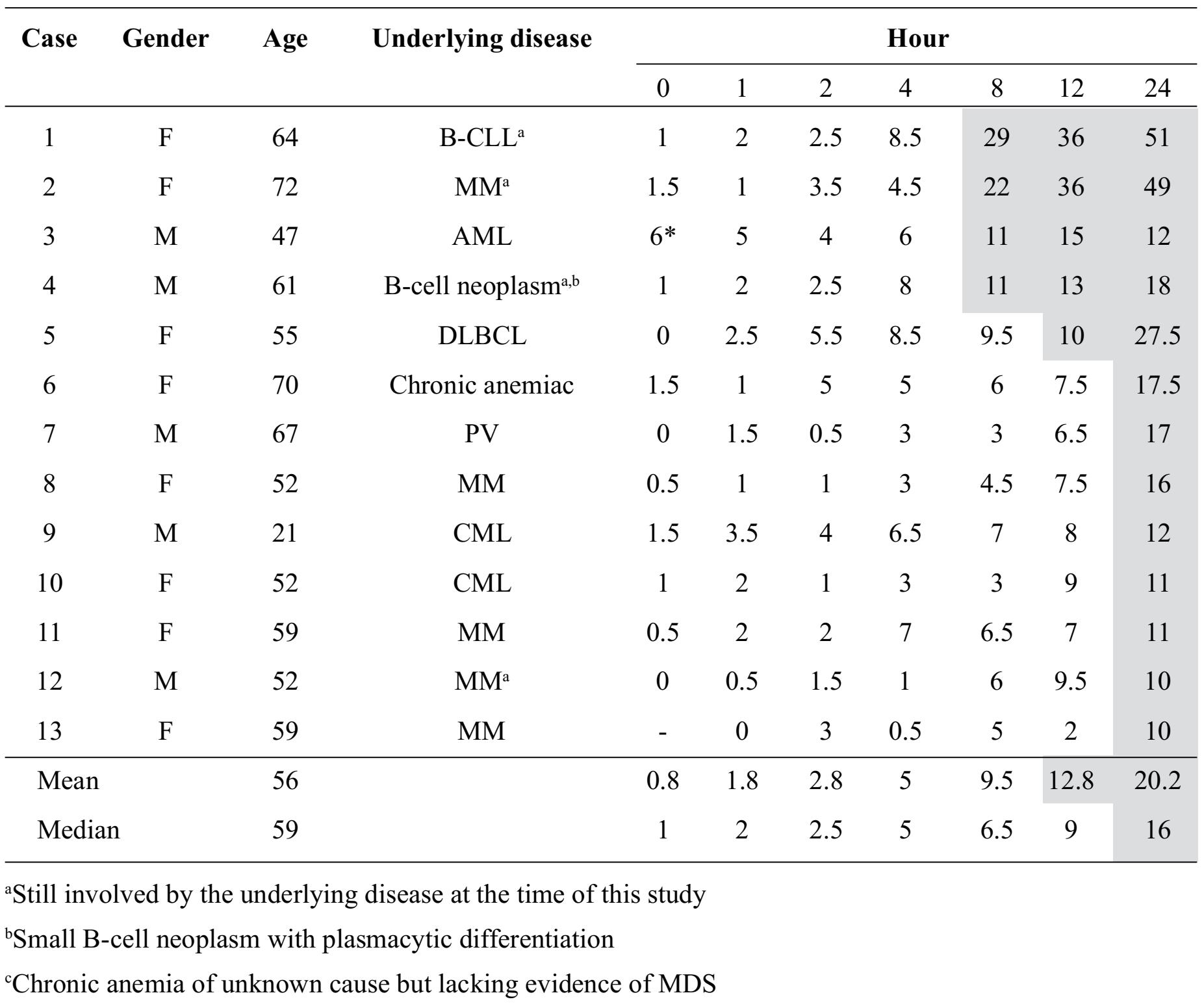
Regarding significant DLNCs observed on marrow aspirate smears (>10% of erythroid precursors), they were initially observed at Hour 8 in 4 cases (30.8%), at Hour 12 in 1 case (7.7%) and at Hour 24 in 8 cases (61.5%). When DLNCs were considered at one particular time interval, the corresponding mean and median percentages from all 13 cases were shown in Table 1. Again, using the 10% cut-off as significant DLNCs, the mean and median percentages of significant DLNCs were initially found on the marrow aspirate smears at Hour 12 (mean 12.8%) and at Hour 24 (median 16%), respectively.
In this study, the information from clotted marrow sections was limited due to insufficient amount of tissue for evaluation. Furthermore, during evaluation of each marrow sample, if the marrow sample contained small number of erythroid component, the changes in DLNCs among all marrow aspirate smears and clotted marrow sections generated from this particular marrow sample appeared to be less obvious than those with more number of erythroid precursors during morphologic assessment. This observation was quite common in the clotted marrow sections as the leftover specimens of clotted marrow frequently obtained less amount of clotted marrow.
Another observation during morphologic assessment was that the quality of marrow aspirate smear staining became worse with time - the longer interval of delayed fixation, the worse staining quality of the smear. Moreover, cell degeneration due to delayed fixation, simulating postmortem change, increased with time and it made morphologic assessment more difficult. Bare nuclei, karyorrhexis and cell debris were increasingly observed with time.
DISCUSSION
From this study, we noticed that the longer the time passed, the more percentages of DLNCs were detected. During morphologic assessments of marrow aspirate smears and clotted marrow sections for DLNCs among erythroid precursors in this study, the percentages of DLNCs in one particular marrow sample might vary at different occasions as different areas were examined. But, eventually, the percentages of DLNCs were increased when time passed in marrow samples from the same case as shown in Table 1.
At Hour 0, DLNCs had been already detected in some cases (9 out of 12 cases with adequate marrow smears; 75% of cases). It is known that a few dysplastic erythroblasts may be seen in normal bone marrow samples, as described by Wang and Glasser [4]. However, the percentage of DLNCs at Hour 0 in this study was likely to be too high in few cases (6% in 1 case and 1.5% in 3 cases); the possibility is that certain kinds of diseases might have an effect on this phenomenon. Further study about the underlying causes is needed to determine whether there are any occult myelodysplastic changes or not.
In this study, the significant DLNCs of erythroid precursors on marrow aspirate smears was initially observed in a subset of cases at 8 hours after delayed fixation of the marrow samples. However, the less significant DLNCs could be observed earlier. Hoffman et al [1] noted that “The diseases affecting the erythroid line would be difficult to interpret after the initial 3-hr. period, when bizarre nuclei appear,” supporting the findings we have shown. In fact, it is reasonable to use the 10% cut-off in this study as we followed the WHO classification (2008) that requires dysplastic changes >10% of any myeloid lineage for diagnosis of unilineage dysplasia. [5] Another important factor of DLNCs in marrow specimens is temperature. Wang and Glasser [4] showed that these nuclear changes were temperature dependent since erythroblasts can maintain their normal nuclear shapes up to 72 hours at 1oC to 6oC, when compared to more number of dyserythropoietic features found at the same intervals if kept at room temperature. In real situations, postmortem erythroid changes might be different from case to case depending on many factors because the temperature of the dead body will gradually decreases until it reaches the room temperature where it is located. In many hospitals, the dead bodies will be placed in the patient wards for 2 hours before moving to pathology department for autopsy examination. The temperature will change during transportation and will be affected by the duration of time which the bodies are kept in the refrigerator while waiting for the examination.
Interestingly, Chisholm and Heerema-McKenney [3] described the relationship between erythroid changes and erythropoietin elevation resulting in “stress dyserythropoiesis.” From their study about erythroid nuclear irregularities in fetal and neonatal autopsies, they also mentioned about the association of increased fetal hemoglobin with dyserythropoiesis in numerous settings, including after severe hemorrhage and myelodysplastic syndrome which is also intriguing but beyond our study.
Bone marrow sections were difficult to evaluate DLNCs, especially in marrow sample parts from the longer intervals of delayed fixation which showed degenerative changes. Morphologic evaluation of dyserythropoiesis on H&E-stained histologic sections was obviously inferior to marrow aspirate smears. PAS stain may help distinguishing erythroid series from myeloid series.
In conclusion, DLNCs do occur and the significant change at 10% cut-off from this study was initially observed at 8 hours after delayed fixation. This phenomenon can interfere with the evaluation of erythroid changes concerning any disease related to dyserythropoiesis when delayed fixation occurs or post-mortem changes at autopsy.
ACKNOWLEDGMENTS
The authors thank all laboratory personnel at Department of Pathology and at bone marrow examination room, Hematology unit, Department of Medicine for their supports.
REFERENCES
1. Hoffman SB, Morrow GW Jr, Pease GL, et al.Rate of cellular autolysis in postmortem bone marrow. Am J Clin Pathol 1964;41:281-6.
2. Findlay AB. Bone marrow change in the post mortem interval. Forens Sci Soc 1977;16;213-8.
3. Chisholm KM, Heerema-McKenney A. Eryth-roid Nuclear Irregularities: a Review of Fetal and Neonatal Autopsies and Correlation with Clinical Features. Ann Clin Lab Sci 2014;44:10-8.
4. Wang L, Glasser L. Spurious dyserythropoiesis. Am J Clin Pathol 2002; 117:57-9.
5. Brunning RD, Porwit A, Orazi A, et al. Myelo-dysplastic syndromes/neoplasms, overview. In: Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC; 2008. p: 88-93.


