Gap Analysis Study before Implementation of Thailand’s National Guidelines for Point of Care Testing at Chiang Rai Province
Nisarat Opartkiattikul MD, PhD, FRCPath (Thailand)1, Surasaak Muenpol Bsc (MT)2
Thawatchai Jaikamwang MD3
1 Faculty of Medicine Siriraj Hospital, Mahidol University, 2 The Bureau of Laboratory Quality Standard,
3 Chiang Rai Provincial Public Health Office
Received 20 April 2016 Accepted; 14 May 2016
ABSTRACT
Background: The Bureau of Laboratory Quality Standards has published Thailand’s National Guidelines for Point-of-Care Testing (POCT) since August 2015. Still yet, the implementation of the guidelines is a great challenge.
Material and Methods: Questionnaires derived from fifty-eight guidelines of the Thailand’s National Guidelines for POCT were created to obtain the opinion towards the importance of the guidelines, the possibility to implement and current compliances to estimate for the gap. Twenty-nine of the representatives from Chiang Rai province which included fifteen medical technologists from community hospitals and fourteen non-laboratory personnel from health promoting hospitals were invited to participate in the study.
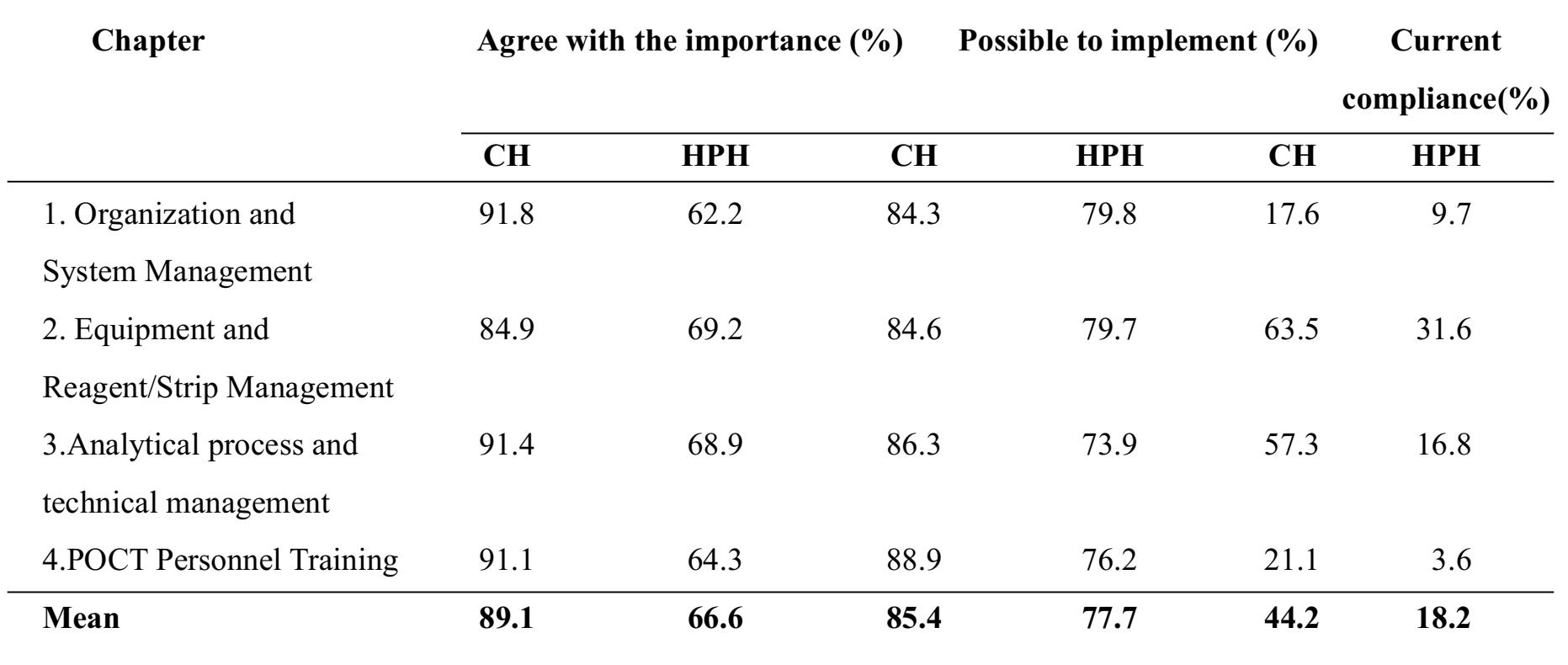
Results: The obtained results showed that 89.1% of the representatives from the community hospitals agreed with the importance of the guidelines, 85.4% thought it possible to implement, and 44.2% claimed present compliance. Among the representatives from the health promoting hospitals, 66.6 % agreed with the importance of the guidelines, 77.7% thought the guidelines possible to implement, 18.2 % claimed current compliance.
Conclusion: The obtained information is useful in planning for the implementation of the guidelines in Chiang Rai province as well as the other provinces in Thailand.
INTRODUCTION
Point of Care Testing (POCT) is defined as medical diagnostic testing performed near the place where a patient is receiving care (1). The aim of using POCT is to improve therapeutic turnaround time (2). These tests are suitable for use in homes, clinics, and hospitals. Among the many kinds of POCT, glucose diagnostics is the most common
use of POCT worldwide (3). The devices have been designed for diabetic screening, supporting the diagnosis of diabetic complications, and treatment monitoring (4). In Thailand, the Ministry of Public Health recently launched a policy that every health promoting hospital has a responsibility to screen and monitor diabetic patients. Accordingly, the quality of the POCT glucose system is essential. The International Organization for Standardization (ISO) has published two standards related to POCT. The ISO 15197 standard is for producing accurate and precise POCT glucose devices (5). ISO 22870 applies to the quality and technical management of POCT within hospitals (6). At the national level, the Bureau of Laboratory Quality Standards (BLQS) has published Thailand’s National Guidelines for POCT since August 2015 (7). There are fifty-eight guidelines divided into four chapters i.e. 1) Organization and System Management, 2) Equipment and Reagent/Strip Management 3) The Analytical Process and Technical Management and 4) POCT Personnel Training. Still yet, the implementation of the guidelines is a great challenge.
In fiscal year 2016, the Chiang Rai Provincial Public Health Office procured a contract for 800,000 POCT glucose strips (with two levels of internal quality control solution and 800 POCT glucose devices); a sufficient supply for every public hospital in its province. In collaboration with the Chiang Rai Provincial Public Health Office, the authors did a study to obtain the opinion of the involved personnel towards the Thailand’s National Guidelines for POCT before implementation of the guidelines in this province.
In October 2016, the representatives from fifteen community hospitals and fourteen health promoting hospitals were invited to attend a one day training program about the new guidelines. All twenty-nine representatives of the hospitals in Cha-ing Rai province were requested to answer the questionnaires during the training program. This study received approval from the Deputy Director of the Chiang Rai Provincial Public Health Office.
MATERIALS AND METHODS
PATIENTS
Before the implementation of Thailand’s National Guidelines for POCT in Chiang Rai province, an opinion study towards the guidelines was performed by collaboration with the Deputy Director of the Chiang Rai Provincial Public Health Office. The author created questionnaires (yes or no response) derived from fifty-eight guidelines in the aspects of important of each guideline, possible to implement and current compliance to estimate for the gap.
RESULT
All fifteen representatives of the community hospitals were medical technologists while all fourteen representatives from health promoting hospital were non-laboratory personnel.
The obtained results showed that 89.1% of the representatives from the fifteen community hospitals agreed with the importance of the guidelines, 85.4% thought it possible to implement, and 44.2% claimed present compliance. Among the representatives from the fourteen health promoting hospitals, 66.6% agreed with the importance of the guidelines, 77.7% thought the guidelines possible to implement, and 18.2% claimed current compliance. The detail information is displayed in Table 1.
Table 1 Detailed information obtained from the survey of fifteen medical technologists associated with community hospitals (CM) and fourteen non-laboratory personnel representing health promoting hospitals (HPH) in Chiang Rai province
DISCUSSION
POCT glucose testing is classified as a “waived test” which can be performed by non-laboratory personnel (8). It is assumed that an erroneous result has no significant risk to patients. However, in some situations, an incorrect result might lead to serious mismanagement (for example, a patient with hypoglycemic coma who showed normal POCT glucose levels). This kind of event can be prevented by a good POCT management system which is the aim of Thailand’s National POCT Guidelines.
Even though, the experts committee who developed the guidelines might try their best to select only important and practical guidelines; the survey results indicated that non-laboratory personnel from HPHs agreed with the importance of the guidelines only 66.6 % of the time, while the medical technologists from CMs agreed with the importance nearly 90% of the time. The most disagreed aspect from HPH representatives was in regards to organization and system management. This reflects the limitation of manpower and management in the typical HPH environment which is usually composed of a fewer number of personnel, such as nurses or other public health staff.
In terms of the practicability of the guidelines, the analytical process and technical management seemed to be more difficult to implement in the HPH setting than in a CH. This finding has encouraged a team approach to operate the POCT system all over country. It is very difficult for non-laboratory personnel to follow the guidelines without any support from laboratory personnel such as clinical pathologist or medical technologist. The gap analysis study also indicated that HPHs compliance was only 18.2% while the CHs compliance was almost 50%. The largest gap from the HPH group was about personnel training. This issue should be solved by clinical pathologists or medical technologists including specialists from the distributor. Another large gap was in regard to the organization and management system. To help fill this gap, the BLQS should produce some quality sample templates of necessary documents and record forms.
In conclusion, the obtained information from the study is useful for the planning and implementation of the guidelines in Chiang Rai province as well as the other provinces in Thailand.
ACKNOWLEDGMENTS
The authors would like to thank Mr. John Waskovsky for editing this manuscript.
REFERENCES
1. Dyhdalo KS, Howanitz PJ, Wilkinson DS, Souers RJ, Jones BA. Documentation of quality control and operator training at point-of-care testing: a College of American Pathologists Q-Probes study of 106 institutions. Arch Pathol Lab Med. 2014;138(11):1444-8.
2. Wagner EA, Yasin B, Yuan S. Point-of-care testing: twenty years’ experience. Lab Medicine. 2008;39(9):560-3.
3. St John A and Price CP. Existing and Emerging Technologies for Point-of-Care Testing. Clin Biochem Rev 2014; 35(3): 155-67.
4. Clinical and Laboratory Standards Institute (CLSI) Point-of-care blood glucose testing in acute and chronic care facilities. Approved Guideline. Third Edition. Wayne, PA, USA: CLSI; 2013. CLSI POCT12-A3.
5. ISO 15197:2015. In vitro diagnostic test sys-tems-requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus.
6. ISO 22870:2006, Point-of-care testing (POCT) — Requirements for quality and competence.
7. BLQS : Thailand’s National Guidelines for Point-of-Care Testing. August 2015.
8. FDA. Guidance for Industry and FDA Staff: Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices. January 30, 2008 available: From http://www.fda.gov/downloads/Medical-Devices/DeviceRegulationand Guidance/Guid-anceDocuments/ucm070890.pdf


